Question: Please EXPLAIN and solve EACH / ALL part(s) in Question #3 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #3!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
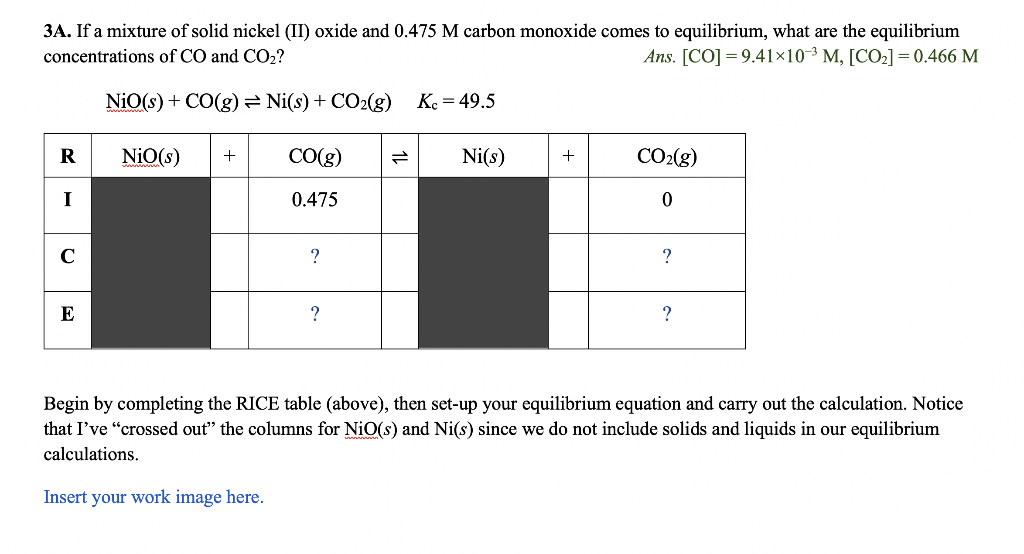
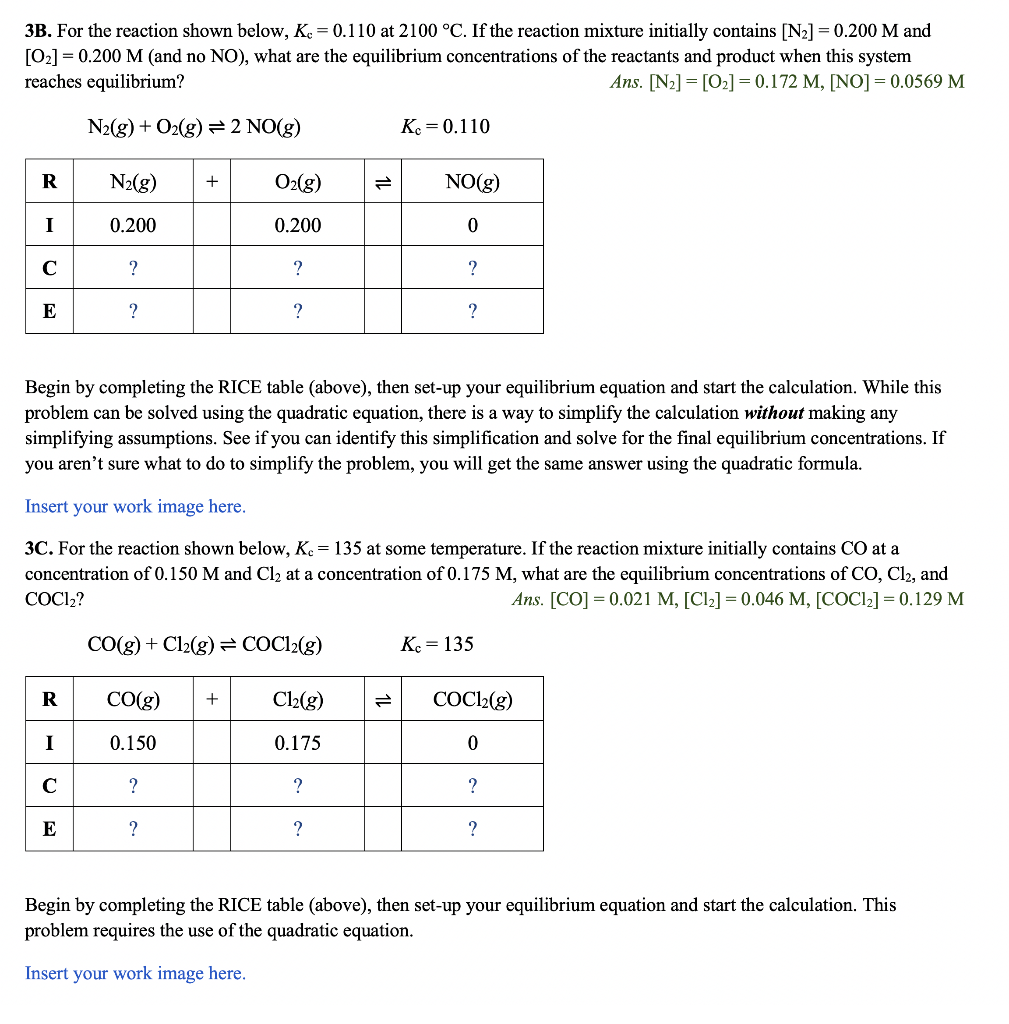
3A. If a mixture of solid nickel (II) oxide and 0.475M carbon monoxide comes to equilibrium, what are the equilibrium concentrations of CO and CO2 ? Ans.[CO]=9.41103M,[CO2]=0.466M NiO(s)+CO(g)Ni(s)+CO2(g)Kc=49.5 Begin by completing the RICE table (above), then set-up your equilibrium equation and carry out the calculation. Notice that I've "crossed out" the columns for NiO(s) and Ni(s) since we do not include solids and liquids in our equilibrium calculations. Insert your work image here. 3B. For the reaction shown below, Kc=0.110 at 2100C. If the reaction mixture initially contains [N2]=0.200M2 and [O2]=0.200M (and no NO ), what are the equilibrium concentrations of the reactants and product when this system reaches equilibrium? Ans.[N2]=[O2]=0.172M,[NO]=0.0569M N2(g)+O2(g)2NO(g)Kc=0.110 Begin by completing the RICE table (above), then set-up your equilibrium equation and start the calculation. While this problem can be solved using the quadratic equation, there is a way to simplify the calculation without making any simplifying assumptions. See if you can identify this simplification and solve for the final equilibrium concentrations. If you aren't sure what to do to simplify the problem, you will get the same answer using the quadratic formula. Insert your work image here. 3C. For the reaction shown below, Kc=135 at some temperature. If the reaction mixture initially contains CO at a concentration of 0.150M and Cl2 at a concentration of 0.175M, what are the equilibrium concentrations of CO,Cl2, and COCl2? Ans.[CO]=0.021M,[Cl2]=0.046M,[COCl2]=0.129M CO(g)+Cl2(g)COCl2(g)Kc=135 Begin by completing the RICE table (above), then set-up your equilibrium equation and start the calculation. This problem requires the use of the quadratic equation. Insert your work image here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


