Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please EXPLAIN and solve EACH / ALL part(s) in Question #4 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #4!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
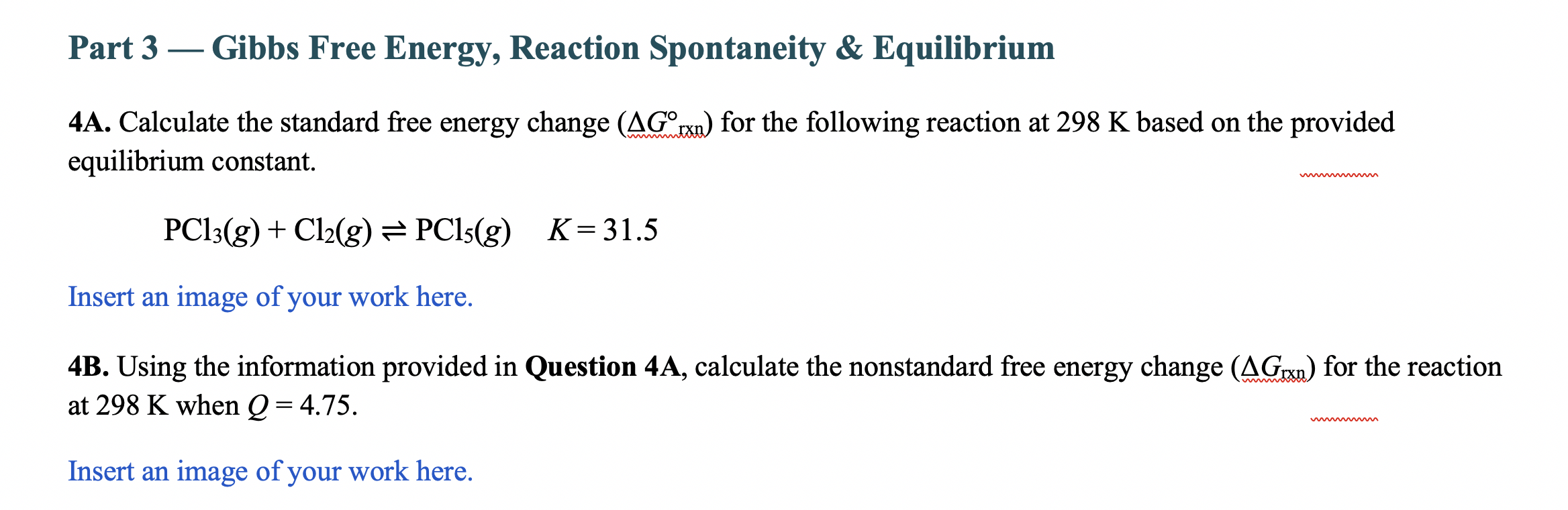
4A. Calculate the standard free energy change (G1xx) for the following reaction at 298K based on the provided equilibrium constant. PCl3(g)+Cl2(g)PCl5(g)K=31.5 Insert an image of your work here. 4B. Using the information provided in Question 4A, calculate the nonstandard free energy change (Grxn) for the reaction at 298K when Q=4.75 Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


