Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please EXPLAIN and solve EACH / ALL part(s) in Question #2 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #2!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
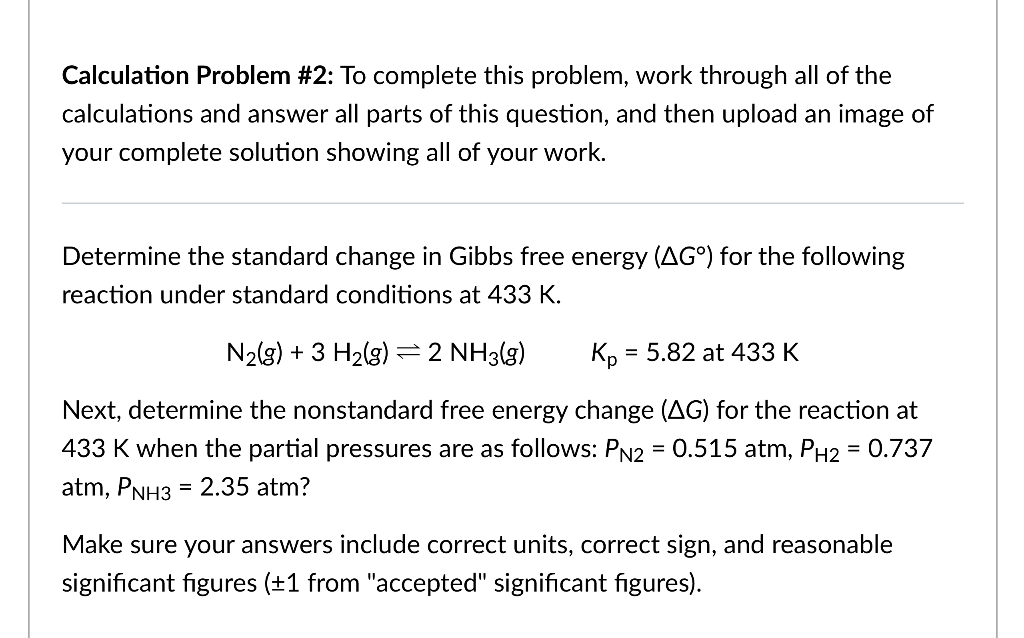
Calculation Problem \#2: To complete this problem, work through all of the calculations and answer all parts of this question, and then upload an image of your complete solution showing all of your work. Determine the standard change in Gibbs free energy (G) for the following reaction under standard conditions at 433K. N2(g)+3H2(g)2NH3(g)Kp=5.82at433K Next, determine the nonstandard free energy change (G) for the reaction at 433K when the partial pressures are as follows: PN2=0.515atm,PH2=0.737 atm,PNH3=2.35atm ? Make sure your answers include correct units, correct sign, and reasonable significant figures ( 1 from "accepted" significant figures)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


