Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain fully You are in charge of a combustion furnace. The furnace is fed an air stream and a methane stream which is combusted
please explain fully 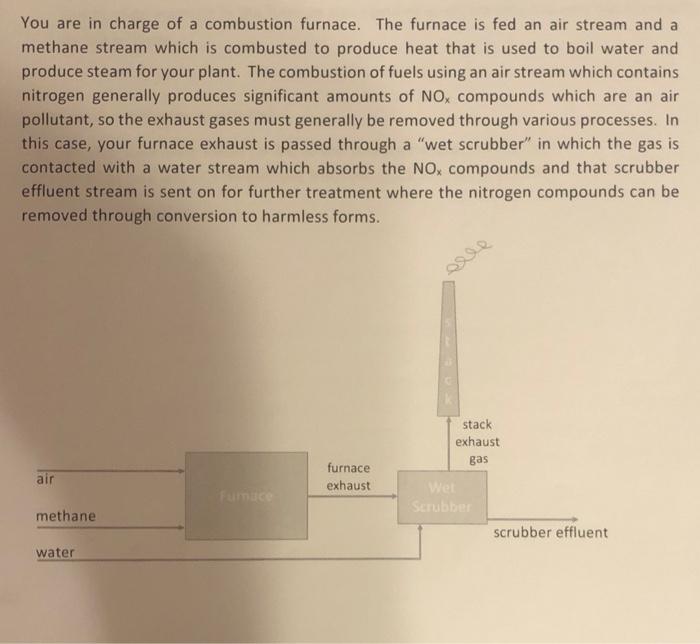

You are in charge of a combustion furnace. The furnace is fed an air stream and a methane stream which is combusted to produce heat that is used to boil water and produce steam for your plant. The combustion of fuels using an air stream which contains nitrogen generally produces significant amounts of NOx compounds which are an air pollutant, so the exhaust gases must generally be removed through various processes. In this case, your furnace exhaust is passed through a "wet scrubber" in which the gas is contacted with a water stream which absorbs the NOx compounds and that scrubber effluent stream is sent on for further treatment where the nitrogen compounds can be removed through conversion to harmless forms. 1. You are feeding 1000mol/hr of methane to the furnace. 2. You are feeding air (79mol%N2 and 21 mol\% O2) at a flow rate such that there is an extra 25 mol\% excess oxygen in the air stream relative to what would be needed stoichiometrically to combust the methane. This feed of excess oxygen is common to ensure complete combustion of the fuel in the furnace. 3. You can assume that all the methane is combusted to form CO2 and H2O through reaction with the oxygen in the furnace. 4. You can assume that 1% of the nitrogen fed to the furnace also reacts with oxygen at the high temperatures to form NO2. 5. The scrubber removes all of the NO2 from the furnace exhaust and enough water is fed to the scrubber so that the concentration of NO2 in the scrubber effluent stays at 2 mol\% (i.e. if the concentration of NO2 were to get too high it would exceed the solubility limit and all of the NO2 would not be removed from the exhaust and it would be sent out the stack). Assume that CO2 is not soluble in the water. 6. The exhaust gas is so hot from the furnace that 5% of the water stream fed to the scrubber is evaporated to form steam and that all of the water formed in the combustion reaction in the furnace also remains as steam which is sent out the stack as water vapor. Answer the following questions: 1. At what rate must water be fed to the scrubber in units of [kg/hr] ? 2. At what rate will CO2 be emitted from the stack in units of [kg/hr] 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


