Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain how to do the molecular and ionic equation as easy as possible The reaction between magnesium nitrate and sodium hydroxide Start bv identifvine
please explain how to do the molecular and ionic equation as easy as possible 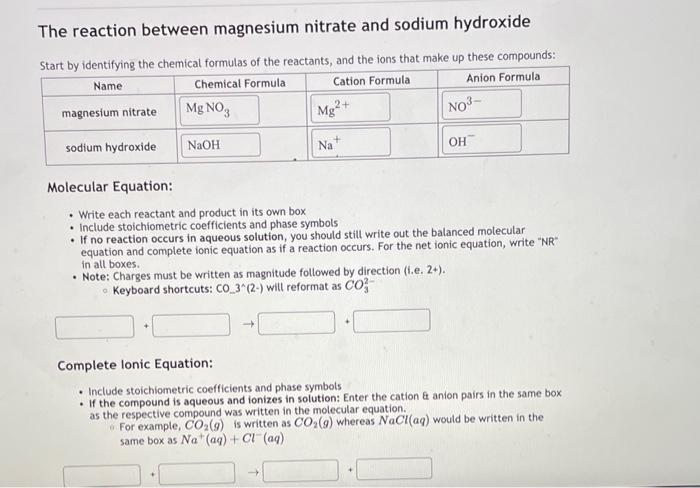
The reaction between magnesium nitrate and sodium hydroxide Start bv identifvine the chemical formulas of the reactants, and the ions that make up these compounds: Molecular Equation: - Write each reactant and product in its own box - Include stoichiometric coefficients and phase symbols - If no reaction occurs in aqueous solution, you should still write out the balanced molecular equation and complete ionic equation as if a reaction occurs. For the net ionic equation, write "NR in all boxes. - Note: Charges must be written as magnitude followed by direction (i.e. 2+ ). Keyboard shortcuts: CO33 (2-) will reformat as CO32 Complete Ionic Equation: - Include stoichiometric coefficients and phase symbols - If the compound is aqueous and ionizes in solution: Enter the cation anion pairs in the same box as the respective compound was written in the molecular equation. For example, CO2(g) is written as CO2(g) whereas NaCl(aq) would be written in the same box as Na+(aq)+Cl(aq) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


