Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain, thank you! 1) An insulated container holds 100.0g of liquid water (H2O,MW=18.02g/mol) at a temperature T=50.0C. An ice cube of mass 10.00g, at
please explain, thank you! 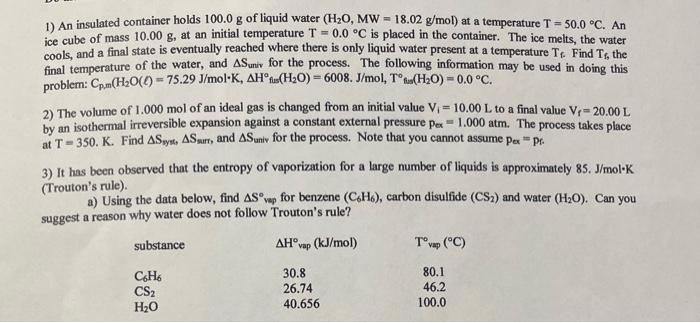
1) An insulated container holds 100.0g of liquid water (H2O,MW=18.02g/mol) at a temperature T=50.0C. An ice cube of mass 10.00g, at an initial temperature T=0.0C is placed in the container. The ice melts, the water cools, and a final state is eventually reached where there is only liquid water present at a temperature Tf. Find Tf, the final temperature of the water, and Suniv for the process. The following information may be used in doing this problem: Cp,m(H2O()=75.29J/molK,H fus (H2O)=6008.J/mol,T fus (H2O)=0.0C. 2) The volume of 1.000mol of an ideal gas is changed from an initial value Vi=10.00L to a final value Vf=20.00L by an isothermal irreversible expansion against a constant external pressure pex =1.000atm. The process takes place at T=350.K. Find Syat,Ssurr, and Suniv for the process. Note that you cannot assume per=p. 3) It has been observed that the entropy of vaporization for a large number of liquids is approximately 85 . J/mol K (Trouton's rule). a) Using the data below, find S vep for benzene (C6H6), carbon disulfide (CS2) and water (H2O). Can you suggest a reason why water does not follow Trouton's rule 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


