Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please explain the steps for using solver in excel to find the velocity. Problem #1 (10 points) The van der Waals (vdW) equation is a
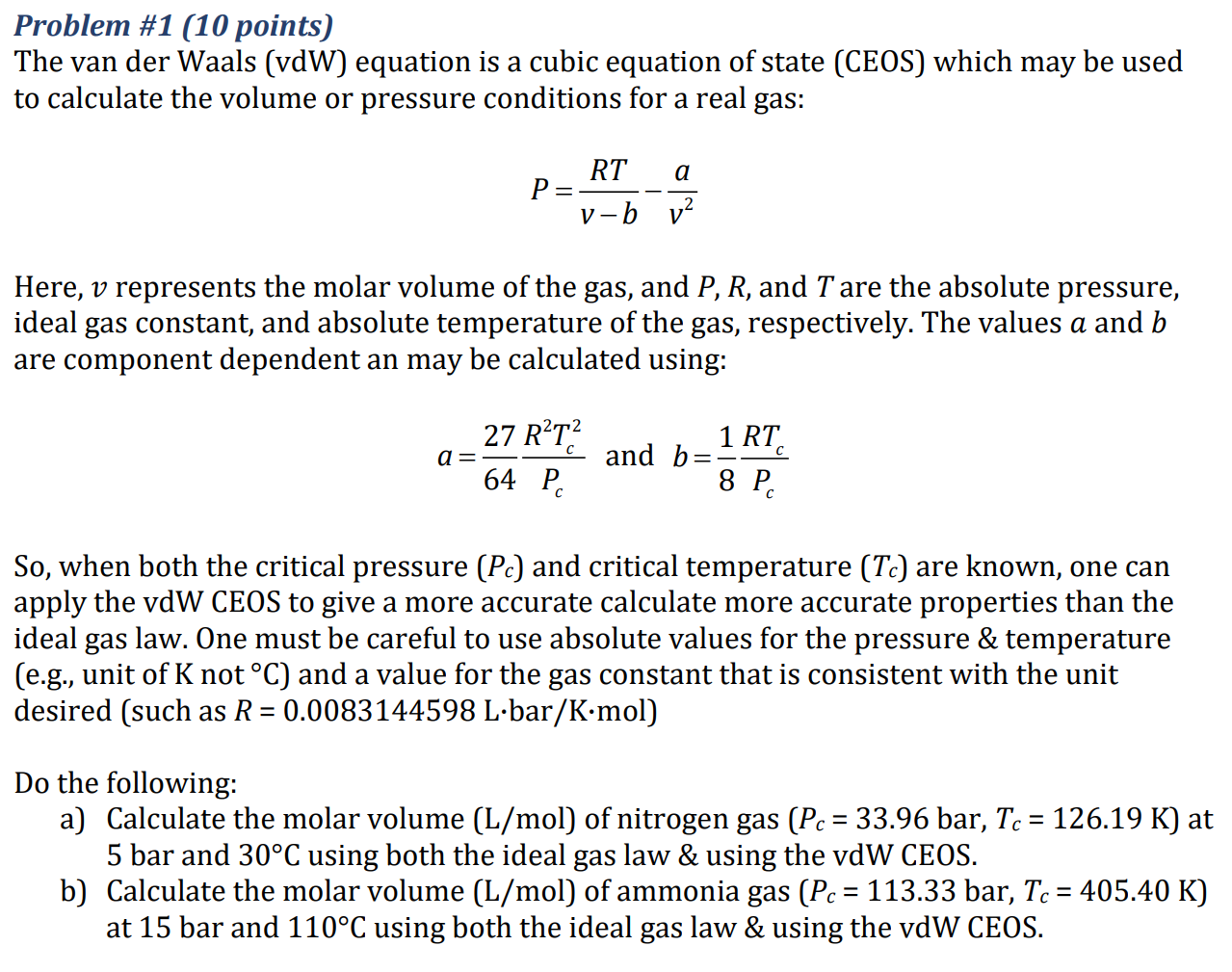
Please explain the steps for using solver in excel to find the velocity.
Problem #1 (10 points) The van der Waals (vdW) equation is a cubic equation of state (CEOS) which may be used to calculate the volume or pressure conditions for a real gas: RT a P= V-b v2 Here, v represents the molar volume of the gas, and P, R, and T are the absolute pressure, ideal gas constant, and absolute temperature of the gas, respectively. The values a and b are component dependent an may be calculated using: 27 RT2 a= 1 RT and b = 8 P 64 P, So, when both the critical pressure (Pc) and critical temperature (TC) are known, one can apply the vdW CEOS to give a more accurate calculate more accurate properties than the ideal gas law. One must be careful to use absolute values for the pressure & temperature (e.g., unit of K not C) and a value for the gas constant that is consistent with the unit desired (such as R = 0.0083144598 Lbar/Kmol) - Do the following: a) Calculate the molar volume (L/mol) of nitrogen gas (Pc = 33.96 bar, Tc = 126.19 K) at 5 bar and 30C using both the ideal gas law & using the vdW CEOS. b) Calculate the molar volume (L/mol) of ammonia gas (Pc = 113.33 bar, Tc = 405.40 K) at 15 bar and 110C using both the ideal gas law & using the vdW CEOSStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


