Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please fast... N-14 k' k Run # [CV] (M) [OH-] (M) Units: Units: 1 2 3 F 10 B D E 1 Time(s) Absorbance [CV]
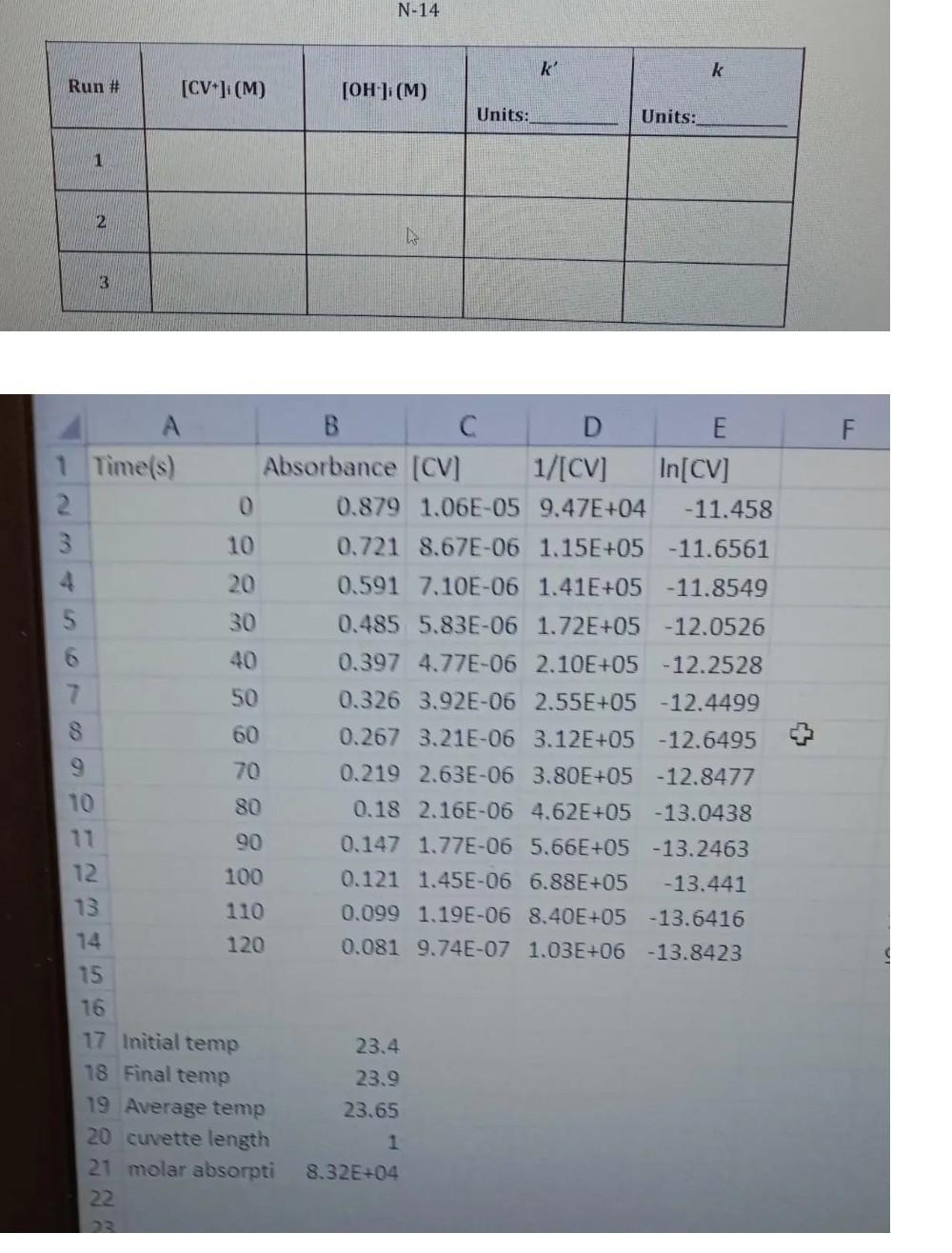
please fast...
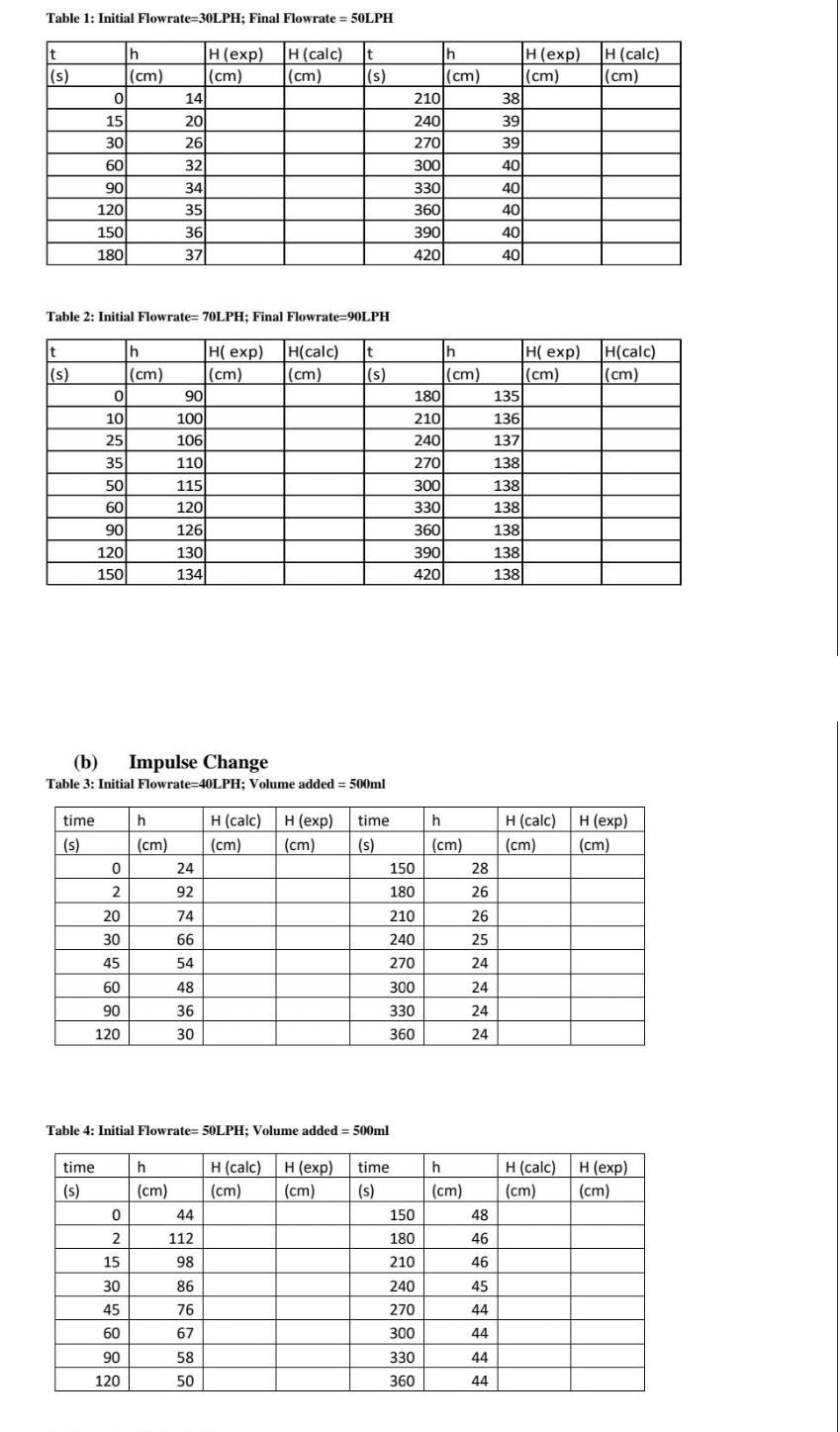
N-14 k' k Run # [CV] (M) [OH-] (M) Units: Units: 1 2 3 F 10 B D E 1 Time(s) Absorbance [CV] 1/[CV] In[CV] 2 0 0.879 1.06E-05 9.47E+04 -11.458 3 0.721 8.67E-06 1.15E+05 -11.6561 4 20 0.591 7.10E-06 1.41E+05 -11.8549 30 0.485 5.83E-06 1.72E+05 -12.0526 40 0.397 4.77E-06 2.10E+05 -12.2528 7 50 0.326 3.92E-06 2.55E+05 -12.4499 8 60 0.267 3.21E-06 3.12E+05 -12.6495 70 0.219 2.63E-06 3.80E+05 -12.8477 10 80 0.18 2.16E-06 4.62E+05 -13.0438 90 0.147 1.77E-06 5.66E+05 -13.2463 12. 100 0.121 1.45E-06 6.88E+05 -13.441 13 110 0.099 1.19E-06 8.40E+05 -13.6416 14 120 0.081 9.74E-07 1.03E+06 -13.8423 15 16 17 Initial temp 23.4 18 Final temp 23.9 19 Average temp 23.65 20 cuvette length 1 21 molar absorpti 8.32E+04 22 m2 Table 1: Initial Flowrate=30LPH; Final Flowrate = 50LPH t h (cm) H(exp) (cm) 14 H(calc) (cm) t (s) (s) h (cm) 210 H(calc) (cm) 0 H (exp) (cm) 38 39 39 15 30 60 $ 90 120 150 180 20 261 32 34 35 36 37 240 270 300 330 360 390 420 40 40 40 40 40 Table 2: Initial Flowrate=70LPH; Final Flowrate=90LPH t (s) H(calc) (cm) t (s) H(calc) (cm) h (cm) 0 10 25 35 H exp) (cm) 90 100 106 110 115 120 126 1301 134 h H exp) (cm) (cm) 1801 135 210 136 240 1371 270 138 300 138) 330 138) 360 138 390 138 420 138) 50 60 90 120 150 (b) Impulse Change Table 3: Initial Flowrate=40LPH; Volume added = 500ml time time h h (cm) 24 H(calc) (cm) H(exp) (cm) H(calc) (cm) H (exp) (cm) (s) (s) (cm) 0 28 150 180 2 92 74 20 30 66 26 26 25 24 24 45 210 240 270 300 330 360 54 48 60 90 120 36 24 24 30 Table 4: Initial Flowrate=50LPH; Volume added = 500ml h time (s) h (cm) H (calc) (cm) H(exp) (cm) H (calc) (cm) H (exp) (cm) (cm) 0 44 48 2 112 46 46 15 98 time (s) 150 180 210 240 270 300 330 360 86 45 30 45 76 44 44 67 60 90 120 58 44 50 44Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


