Answered step by step
Verified Expert Solution
Question
1 Approved Answer
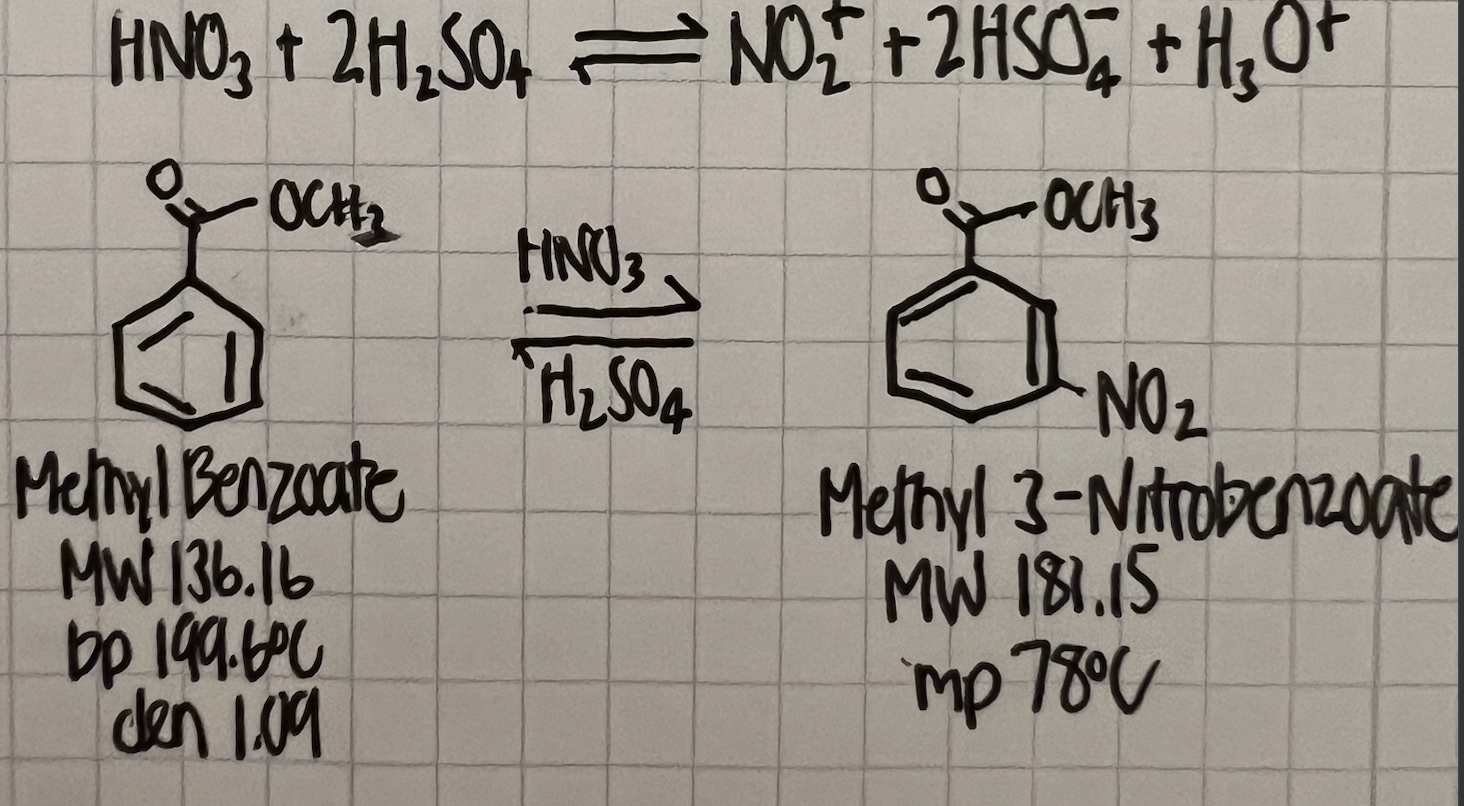
Please find the percent yield of methyl 3-nitrobenzoate given the following (this is the nitration of methyl benzoate using nitric and sulfuric acids): Methyl Benzoate
Please find the percent yield of methyl 3-nitrobenzoate given the following (this is the nitration of methyl benzoate using nitric and sulfuric acids):
Methyl Benzoate Used: 1.011g
Methyl 3-Nitrobenzoate Obtained: 0.445g
Molecular Weight of Methyl Benzoate: 136.16g/mol
Molecular Weight of Methyl 3-Nitrobenzoate: 181.15g/mol
HNO3+2H2SO4NO2++2HSO4+H3OO++1CH3H2SO4HNO3Melhyl3-NitrobenzzateMW181.15MP78C
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


