Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please give a step bu step Part B. Determination of Cirric Acid in Soda. Add precisely 2.00mL of soda to a clean Erlenmeyer flask. Add
please give a step bu step 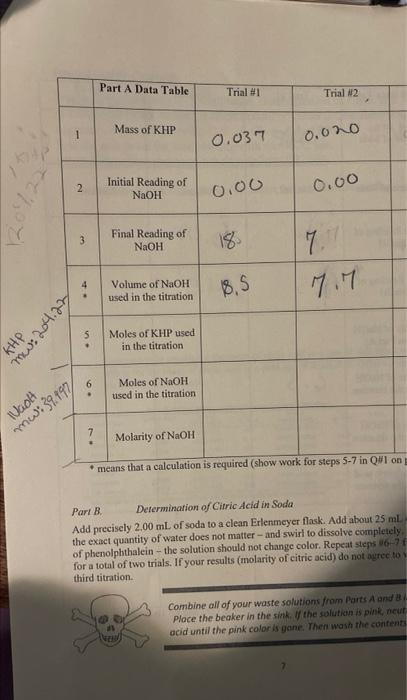
Part B. Determination of Cirric Acid in Soda. Add precisely 2.00mL of soda to a clean Erlenmeyer flask. Add about 25mL. the exact quantity of water does not matter - and swiri to dissolve completely. of phenolphthalein - the solution should not change color. Repeat stepss 667 f for a total of two trials. If your results (molarity of citric acid) do not agrec to) third titration. Combine all of your waste solutions from Parts A and at. Ploce the beaker in the sink, Uf the solution is pink, neut acid until the pink color is gane. Then wash the contents Part B. Determination of Cirric Acid in Soda. Add precisely 2.00mL of soda to a clean Erlenmeyer flask. Add about 25mL. the exact quantity of water does not matter - and swiri to dissolve completely. of phenolphthalein - the solution should not change color. Repeat stepss 667 f for a total of two trials. If your results (molarity of citric acid) do not agrec to) third titration. Combine all of your waste solutions from Parts A and at. Ploce the beaker in the sink, Uf the solution is pink, neut acid until the pink color is gane. Then wash the contents 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


