Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please give correct answer for all and show work for all thank you :) mass number Find the chemical formula and the formula mass for
please give correct answer for all and show work for all thank you :) 
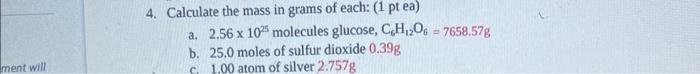


mass number Find the chemical formula and the formula mass for aluminum sulfate. chemical formula: AI2(SO4)3 Formula mass: 342.15g/mol 4. Calculate the mass in grams of each: (1 pt ea) a. 2.561025 molecules glucose, C6H12O6=7658.57g b. 25.0 moles of sulfur dioxide 0.39g c. 1.00 atom of silver 2.757g 5. Calculate the number of moles for each: ( 1pt ea) a. 26.5 grams of copper (II) nitrite 0.141mol b. 9.85 grams of nitrogen gas 0.352mol 2001024 molecules of hydrogen gas 6.695mol 6. Calculate the number of particles for the following: (1pt ea) a. 43.5 grams calcium chloride 2.361023 particles CaCl2 b. 20.0 moles hydrogen gas 1.201025 particles H2 c. 50.0 grams of carbonic acid 4.851023 particles H2CO3 
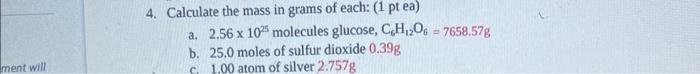


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


