Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please handwrite your solution and show every step thank you! Problem 1 Ethyl benzene (EB) is produced by the reaction between ethylene (E) and benzene
Please handwrite your solution and show every step thank you!
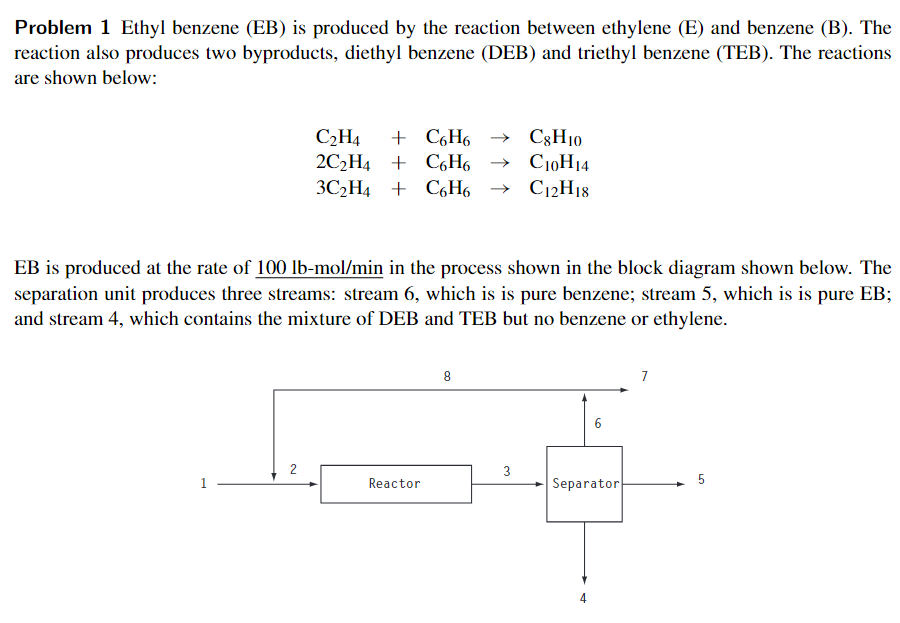
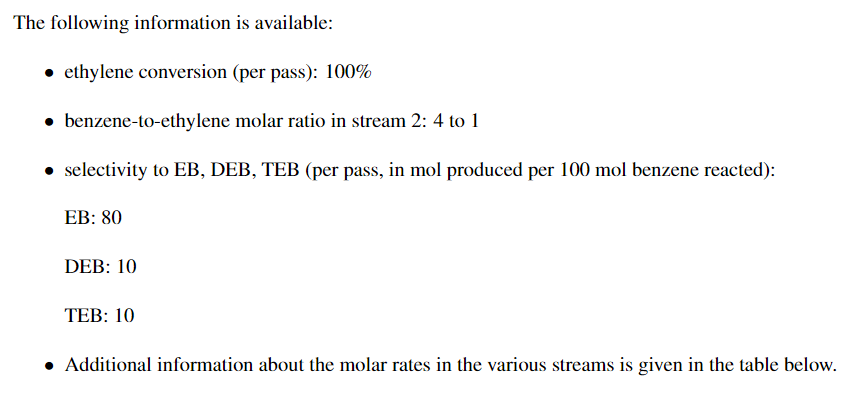
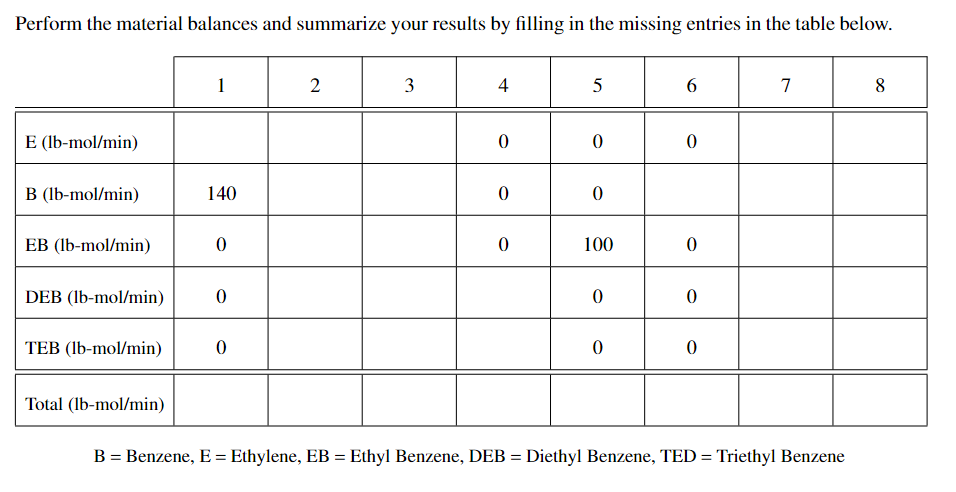
Problem 1 Ethyl benzene (EB) is produced by the reaction between ethylene (E) and benzene (B). The reaction also produces two byproducts, diethyl benzene (DEB) and triethyl benzene (TEB). The reactions are shown below: C2H4 + C6H6 C8H10 2C2H4 + C6H6 C10H14 3C2H4 + C6H6 + C6H6 + C12H18 EB is produced at the rate of 100 lb-mol/min in the process shown in the block diagram shown below. The separation unit produces three streams: stream 6, which is is pure benzene; stream 5, which is is pure EB; and stream 4, which contains the mixture of DEB and TEB but no benzene or ethylene. 8 7 6 2 3 1 Reactor 5 Separator 4 The following information is available: ethylene conversion (per pass): 100% benzene-to-ethylene molar ratio in stream 2: 4 to 1 selectivity to EB, DEB, TEB (per pass, in mol produced per 100 mol benzene reacted): EB: 80 DEB: 10 TEB: 10 Additional information about the molar rates in the various streams is given in the table below. Perform the material balances and summarize your results by filling in the missing entries in the table below. 1 2 3 4 5 6 7 8 E (lb-mol/min) 0 0 0 B (lb-mol/min) 140 0 0 EB (lb-mol/min) 0 0 100 0 DEB (Ib-mol/min) 0 0 0 TEB (lb-mol/min) 0 0 0 Total (lb-mol/min) B = Benzene, E = Ethylene, EB = Ethyl Benzene, DEB = Diethyl Benzene, TED = Triethyl Benzene = = =Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


