Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help ! 0.928 46.668g 1. Mass of sodium carbonate, 2 Describe what happens when the solutions are mixed. 3 Mass of watch glass, g
Please help ! 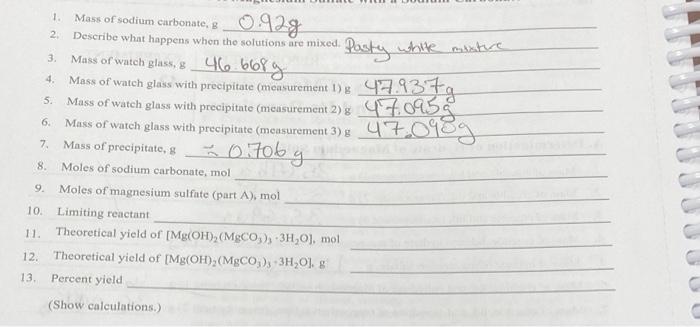
0.928 46.668g 1. Mass of sodium carbonate, 2 Describe what happens when the solutions are mixed. 3 Mass of watch glass, g 4. Mass of watch 5. Mass of watch glass with 6. Mass of watch glass with precipitate (measurement 3) 7. Mass of precipitate, & 8. Moles of sodium carbonate, mol 9. Moles of magnesium sulfate (part A), mol 10. Limiting reactant 11. Theoretical yield of [Mg(OH),(MgCO), 3H,0), mol 12. Theoretical yield of [Mg(OH),(MgCO3), -3H-0). g 13 Percent yield 1 Pasty while misture o glass with precipitate (measurement !) 479379 o precipitate (miensurement 278 47.0955 47.098g x 0.70bg C C (Show calculations.) UU 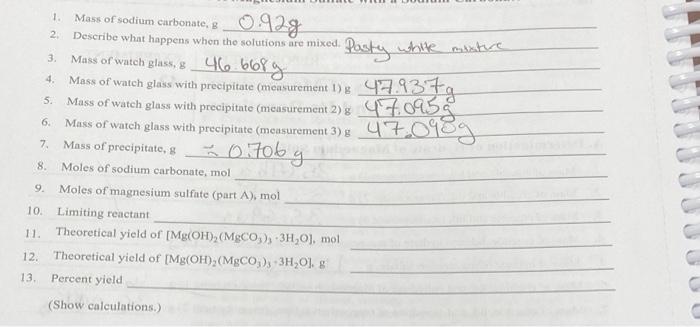
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


