Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help!!!!! A reaction in which A,B, and C react to form products is zero order in A, one-half order in B, and second order
please help!!!!! 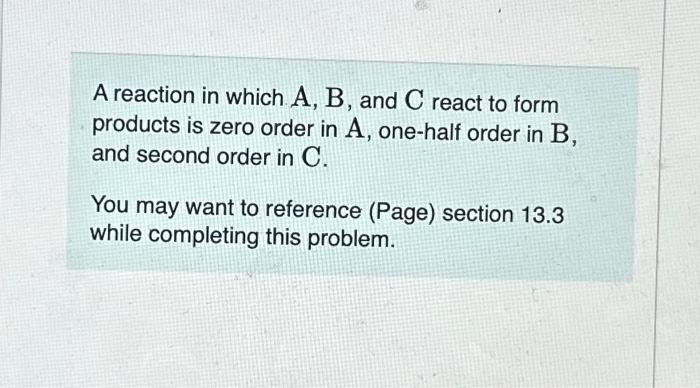

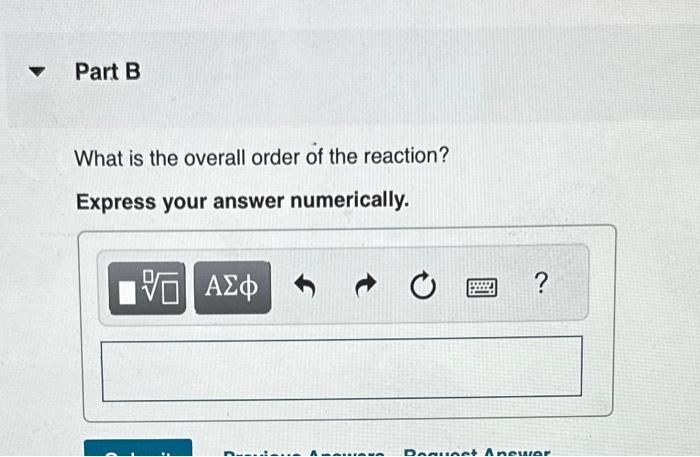
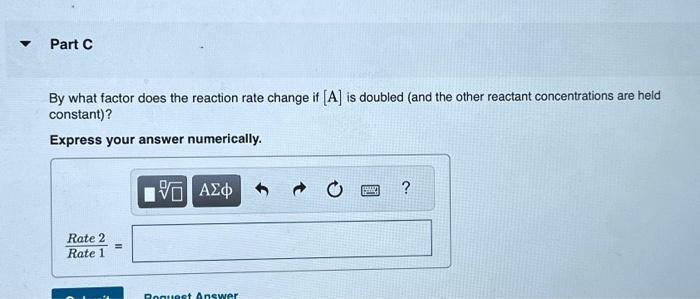
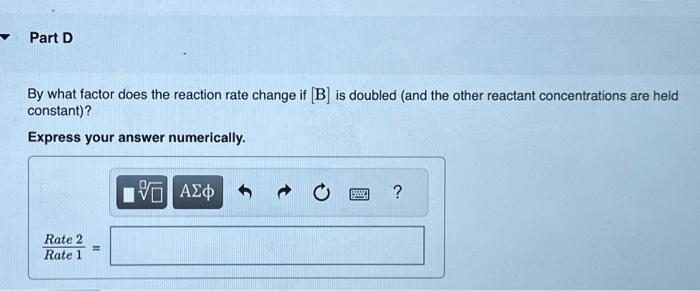
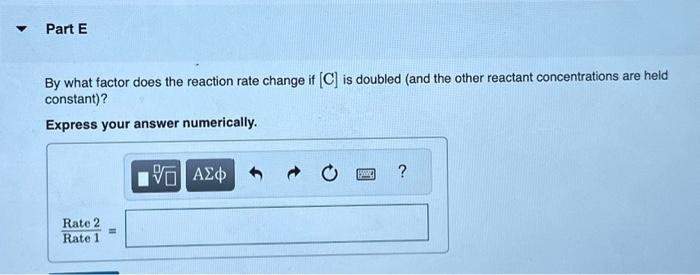
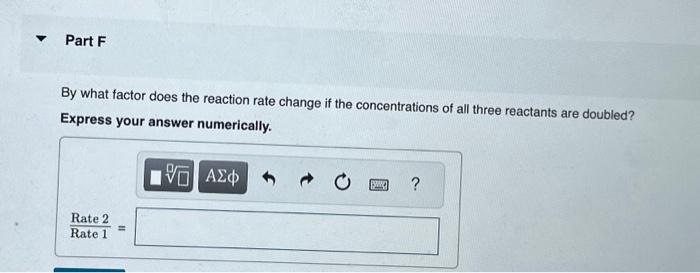
A reaction in which A,B, and C react to form products is zero order in A, one-half order in B, and second order in C. You may want to reference (Page) section 13.3 while completing this problem. Write a rate law for the reaction. Rate=k[A][B]2[C]1/2Rate=k[B][C]1/2Rate=k[B]1/2[C]2Rate=k[A][B]1/2[C] Correct The rate law is used to express the relationship betwe reactant: What is the overall order of the reaction? Express your answer numerically. By what factor does the reaction rate change if [A] is doubled (and the other reactant concentrations are held constant)? Express your answer numerically. By what factor does the reaction rate change if [B] is doubled (and the other reactant concentrations are held constant)? Express your answer numerically. By what factor does the reaction rate change if [C] is doubled (and the other reactant concentrations are held constant)? Express your answer numerically. By what factor does the reaction rate change if the concentrations of all three reactants are doubled? Express your answer numerically 






Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


