please help answer the following questions in the picture above (percent error). thank you
I am not sure if you will need these other 3 pictures, but i will post them just in case!
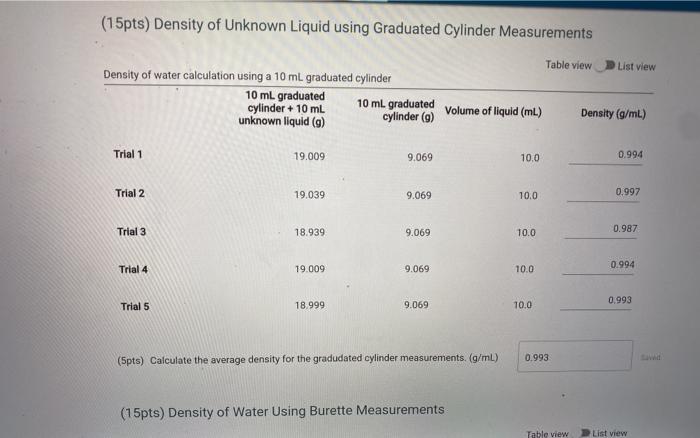
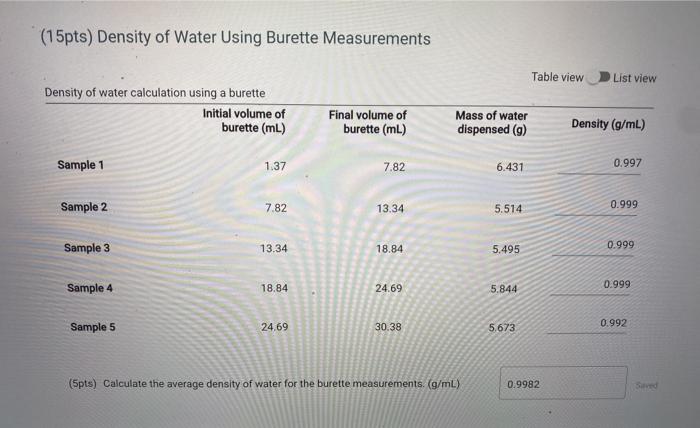
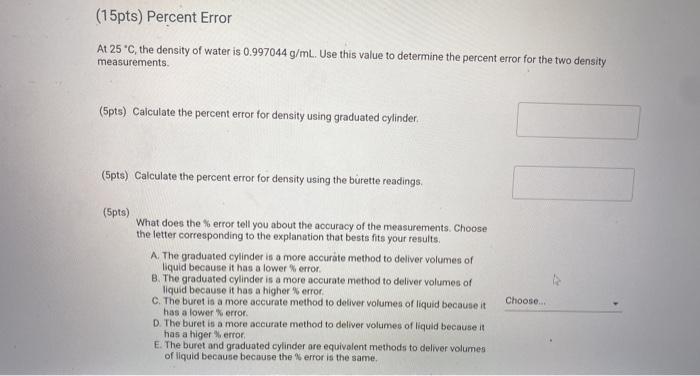
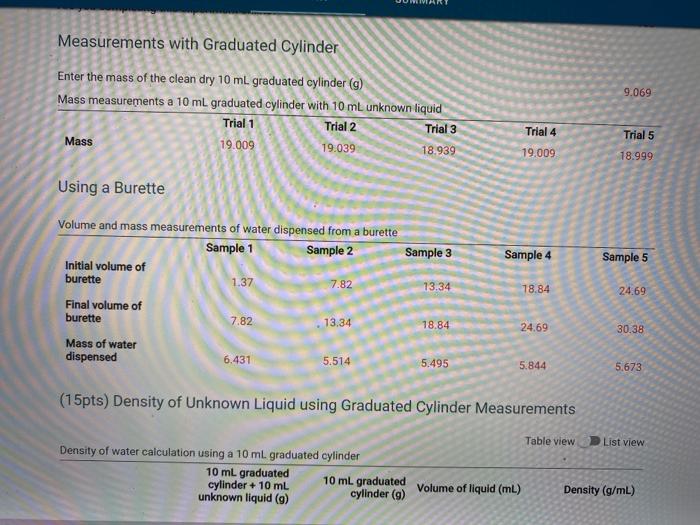
(15pts) Percent Error At 25 C, the density of water is 0.997044 g/mL. Use this value to determine the percent error for the two density measurements (5pts) Calculate the percent error for density using graduated cylinder (5pts) Calculate the percent error for density using the burette readings, (5pts) What does the error tell you about the accuracy of the measurements. Choose the letter corresponding to the explanation that besta fits your results. A The graduated cylinder is a more accurate method to deliver volumes of liquid because it has a lower error B. The graduated cylinder is a more accurate method to deliver volumes of liquid because it has a higher error C. The buret is a more accurate method to deliver volumes of liquid because it has a lower error D. The buret is a more accurate method to deliver volumes of liquid because it has a higer error E. The buret and graduated cylinder ore equivalent methods to deliver volumes of liquid because because the error is the same Choose... Measurements with Graduated Cylinder Enter the mass of the clean dry 10 mL graduated cylinder (g) Mass measurements a 10 mL graduated cylinder with 10 mL unknown liquid Trial 1 Trial 2 Trial 3 Mass 19.009 19.039 18.939 9.069 Trial 4 Trial 5 19.009 18.999 Using a Burette Sample 3 Sample 4 Sample 5 Volume and mass measurements of water dispensed from a burette Sample 1 Sample 2 Initial volume of burette 1.37 7.82 Final volume of burette 7.82 13.34 Mass of water dispensed 6.431 5.514 13.34 78.84 24.69 18.84 24.69 30.38 5.495 5.844 5.673 (15pts) Density of Unknown Liquid using Graduated Cylinder Measurements Table view D List view Density of water calculation using a 10 ml graduated cylinder 10 ml graduated 10 ml graduated cylinder + 10 mL unknown liquid (9) cylinder (9) Volume of liquid (ml) Density (g/mL) (15pts) Density of Unknown Liquid using Graduated Cylinder Measurements Table view List view Density of water calculation using a 10 ml graduated cylinder 10 ml graduated cylinder + 10 ml 10 ml graduated unknown liquid (9) cylinder (9) Volume of liquid (mL) Density (g/ml) Trial 1 19.009 9.069 10.0 0.994 Trial 2 19.039 9.069 10.0 0.997 Trial 3 18.939 9.069 10.0 0.987 Trial 4 19.009 9.069 10.0 0.994 0.993 Trial 5 18.999 9.069 10.0 (5pts) Calculate the average density for the gradudated cylinder measurements. (g/ml) 0.993 (15pts) Density of Water Using Burette Measurements Table view List view (15pts) Density of Water Using Burette Measurements Table view List view Density of water calculation using a burette Initial volume of burette (mL) Final volume of burette (mL) Mass of water dispensed (9) Density (g/mL) Sample 1 1.37 7.82 6.431 0.997 Sample 2 7.82 13.34 5.514 0.999 Sample 3 13.34 18.84 5.495 0.999 Sample 4 18.84 24.69 5.844 0.999 Sample 5 24.69 30.38 5.673 0.992 (5pts) Calculate the average density of water for the burette measurements. (g/mL) 0.9982
 please help answer the following questions in the picture above (percent error). thank you
please help answer the following questions in the picture above (percent error). thank you I am not sure if you will need these other 3 pictures, but i will post them just in case!
I am not sure if you will need these other 3 pictures, but i will post them just in case!