Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help Ap Chemistry Specific Heat Thank you Date Carbon Copy lab Specific Heat Capacity measures how much energy it takes to raise the temperature
please help
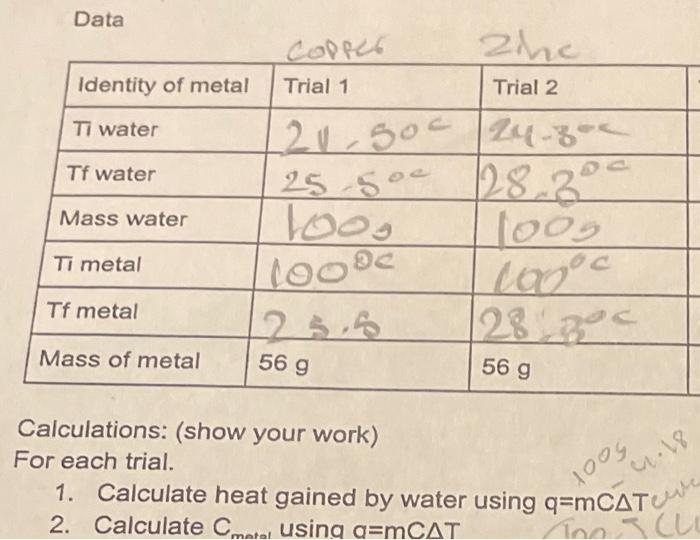
Date Carbon Copy lab Specific Heat Capacity measures how much energy it takes to raise the temperature of a substance 1K (which is the same as 1 Celsius). We know this qualitatively as how quickly a substance heats up or cools down. In this lab you will calculate the specific heat of 2 different metals. Materials: 2 styrofoam cups, 1600mL beaker, 100mL graduated cylinder, 1 digital thermometer Metals in a boiling water bath: Zn,Cu, Steel, Al Procedure: 1. Measure either 150mL of water or 250mL of water using a graduated cylinder 2. Place water into styrofoam cup and take/record the temperature of the water 3. From the boiling water bath take 1 metal and place into your water (the Al goes into the 250mL, the rest go into 150mL ) 4. Stir the water in the cup and record the highest temperature. It may take several minutes. 5. Remove the metal from the cup and return to the instructor. 6. Repeat steps 1-5 until you have 2 trials for 2 different metals. Data Calculations: (show your work) For each trial. 1. Calculate heat gained by water using q=mCT 2. Calculate Cmatal using a=mCT Questions: 1. Why do the metals have different sized cylinders? 2. How do we apply the First Law of Thermodynamics in this lab? 3. Look up the accepted specific heat values for your metals. Calculate your percent error based on what you find Ap Chemistry
Specific Heat
Thank you 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


