Question
PLEASE HELP ASAP. I'm Stuck Using the reaction chemistry and kinetics presented in Example 4.7, change the feed concentration of NO from 5% to 5
PLEASE HELP ASAP. I'm Stuck
Using the reaction chemistry and kinetics presented in Example 4.7, change the feed concentration of NO from 5% to 5 ppm, and examine the effect on the concentration profile for ethane in the PFR at 1050 K. Assume the feed consists only of ethane and NO, and that the pressure and volumetric flow rate are the same as in example 4.7. Explain the effect of the feed NO concentration on the ethane conversion.
Note: Include MATLAB plot in solution. use MATLAB for plot (please include code script in the solution). Include MATLAB plot in solution. 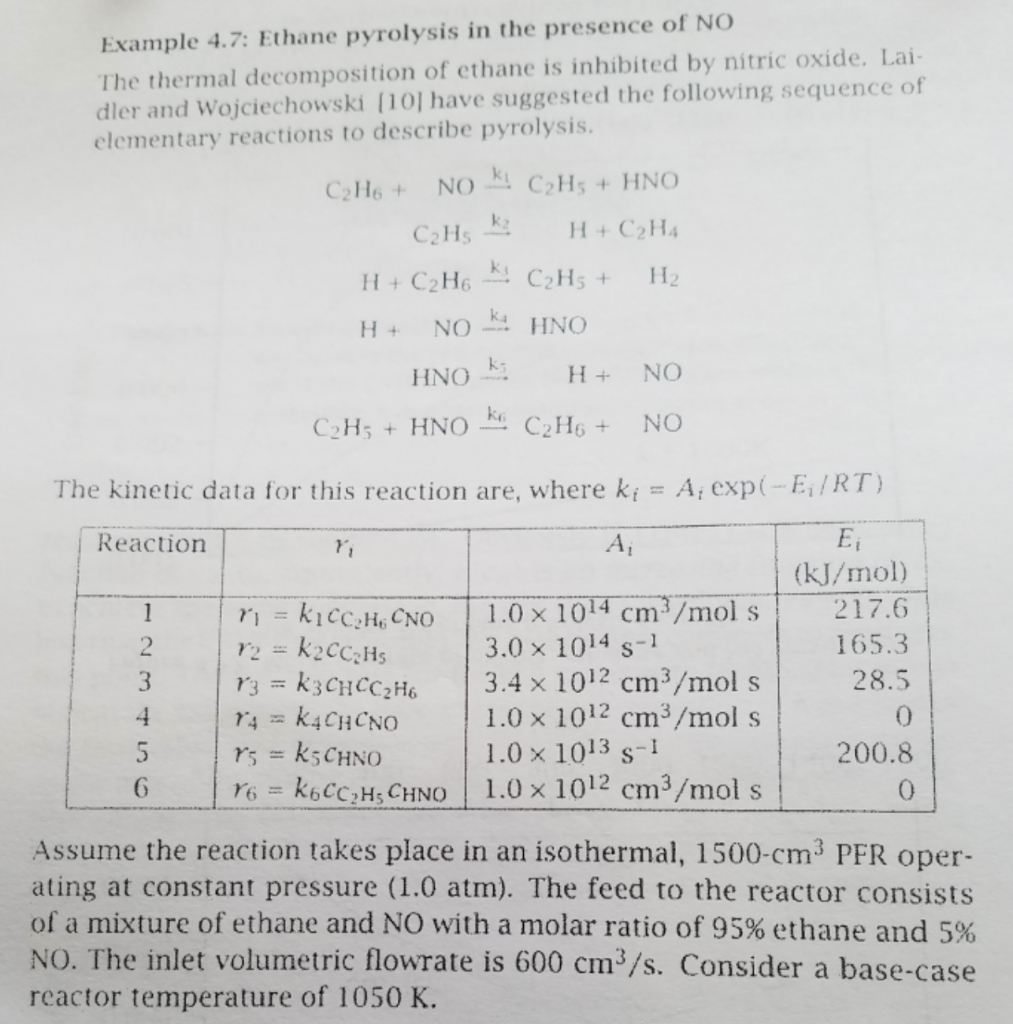
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


