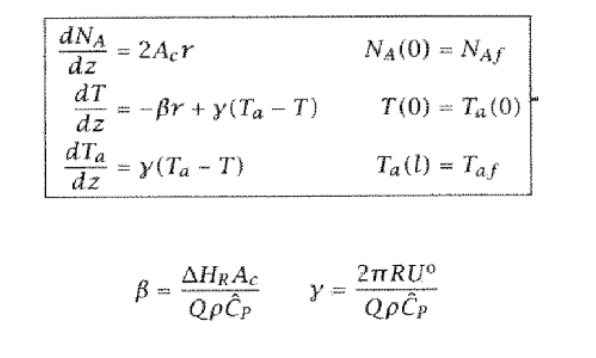PLEASE HELP ASAP. You can plot anywhere, just make sure to include screenshots of the equations and graphs used. Figure 6.38 and Table 6.6 are below for reference.
TABLES AND FIGURES BELOW FOR REFERENCE.
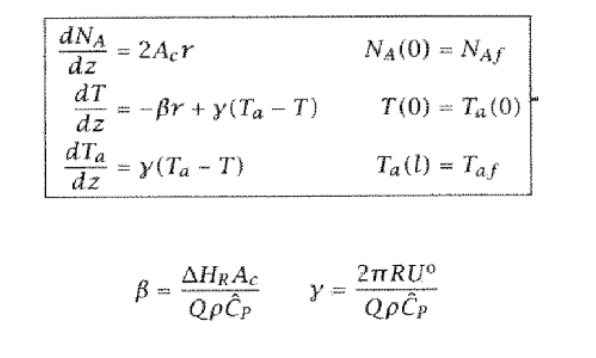
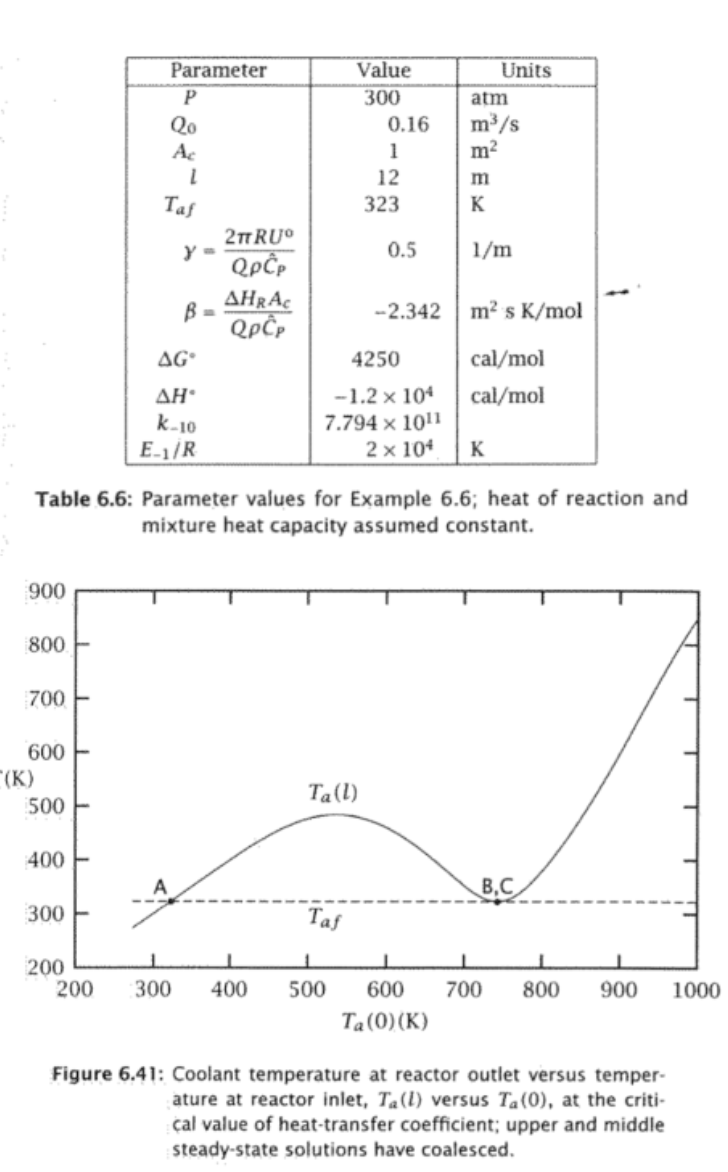
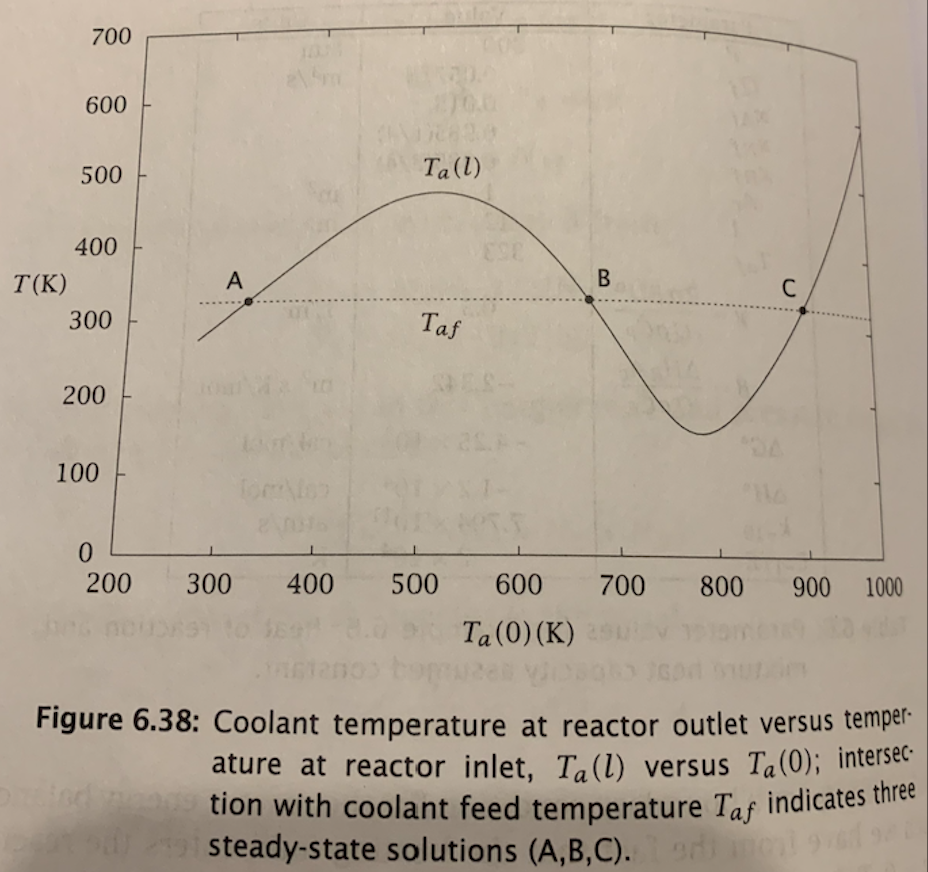
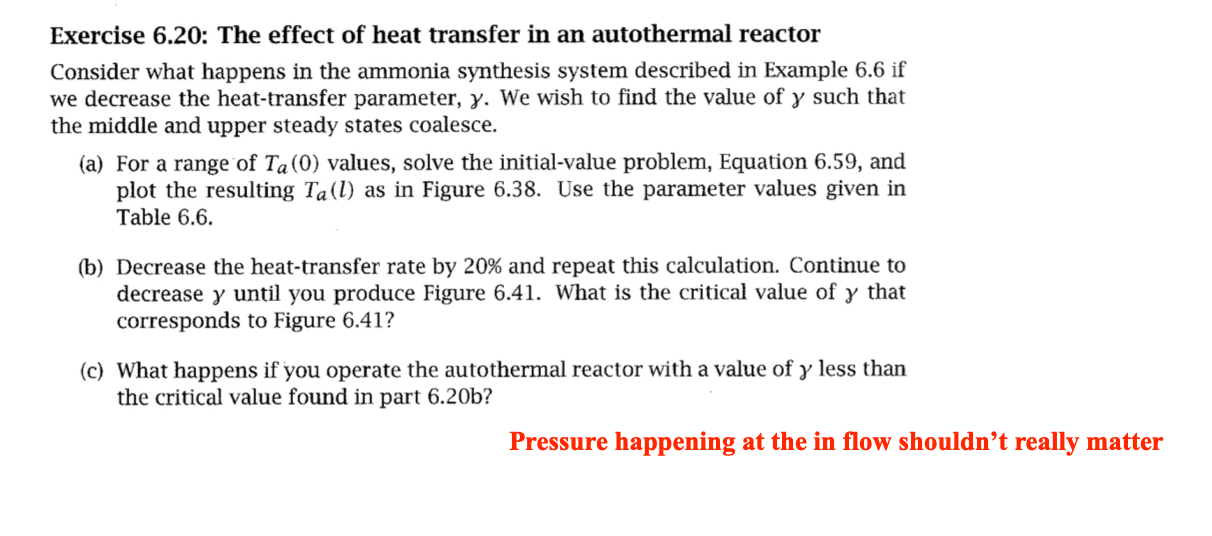
Exercise 6.20: The effect of heat transfer in an autothermal reactor Consider what happens in the ammonia synthesis system described in Example 6.6 if we decrease the heat-transfer parameter, . We wish to find the value of such that the middle and upper steady states coalesce. (a) For a range of Ta(0) values, solve the initial-value problem, Equation 6.59, and plot the resulting Ta(l) as in Figure 6.38. Use the parameter values given in Table 6.6. (b) Decrease the heat-transfer rate by 20% and repeat this calculation. Continue to decrease until you produce Figure 6.41. What is the critical value of y that corresponds to Figure 6.41? (c) What happens if you operate the autothermal reactor with a value of less than the critical value found in part 6.20b ? Pressure happening at the in flow shou dzdNA=2AcrdzdT=r+(TaT)dzdTa=(TaT)NA(0)=NAfT(0)=Ta(0)Ta(l)=Taf =QC^PHRAc=QC^P2RU Table 6.6: Parameter values for Example 6.6 ; heat of reaction and mixture heat capacity assumed constant. Figure 6.41: Coolant temperature at reactor outlet versus temperature at reactor inlet, Ta(l) versus Ta(0), at the critical value of heat-transfer coefficient; upper and middle steady-state solutions have coalesced. Figure 6.38: Coolant temperature at reactor outlet versus temperature at reactor inlet, Ta(l) versus Ta(0); intersec tion with coolant feed temperature Taf indicates three steady-state solutions (A,B,C). Exercise 6.20: The effect of heat transfer in an autothermal reactor Consider what happens in the ammonia synthesis system described in Example 6.6 if we decrease the heat-transfer parameter, . We wish to find the value of such that the middle and upper steady states coalesce. (a) For a range of Ta(0) values, solve the initial-value problem, Equation 6.59, and plot the resulting Ta(l) as in Figure 6.38. Use the parameter values given in Table 6.6. (b) Decrease the heat-transfer rate by 20% and repeat this calculation. Continue to decrease until you produce Figure 6.41. What is the critical value of y that corresponds to Figure 6.41? (c) What happens if you operate the autothermal reactor with a value of less than the critical value found in part 6.20b ? Pressure happening at the in flow shou dzdNA=2AcrdzdT=r+(TaT)dzdTa=(TaT)NA(0)=NAfT(0)=Ta(0)Ta(l)=Taf =QC^PHRAc=QC^P2RU Table 6.6: Parameter values for Example 6.6 ; heat of reaction and mixture heat capacity assumed constant. Figure 6.41: Coolant temperature at reactor outlet versus temperature at reactor inlet, Ta(l) versus Ta(0), at the critical value of heat-transfer coefficient; upper and middle steady-state solutions have coalesced. Figure 6.38: Coolant temperature at reactor outlet versus temperature at reactor inlet, Ta(l) versus Ta(0); intersec tion with coolant feed temperature Taf indicates three steady-state solutions (A,B,C)