Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help Consider the following data for gallium: atomic mass 69.723 g mol electronegativity 1.81 electron affinity kJ 28.9 mol ionization energy 578.8 mol heat
Please help 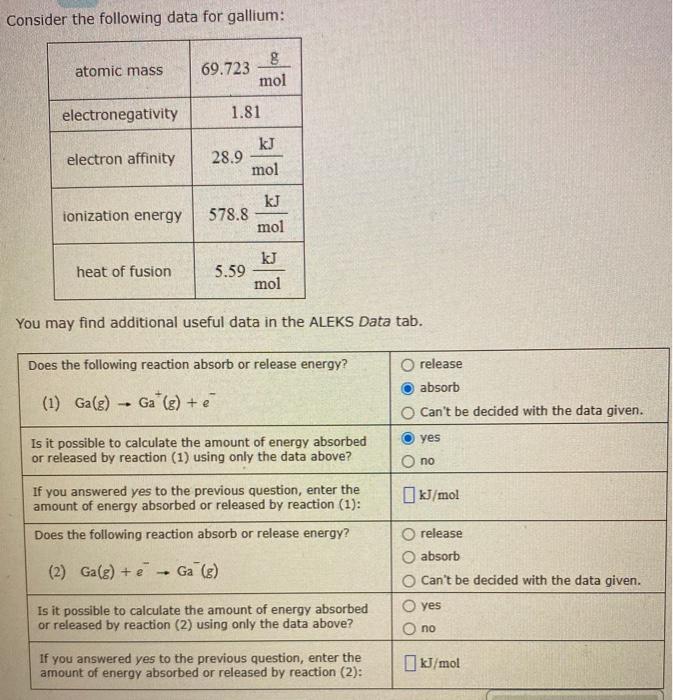
Consider the following data for gallium: atomic mass 69.723 g mol electronegativity 1.81 electron affinity kJ 28.9 mol ionization energy 578.8 mol heat of fusion 5.59 kJ mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? O release absorb O Can't be decided with the data given. (1) Ga(g) Ga (g) + yes Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? O no If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): kJ/mol Does the following reaction absorb or release energy? release O absorb Can't be decided with the data given. (2) Ga(g) + e - Ga (8) yes Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? no If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): kJ/mol 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


