Question: please help Dinitrogen pentoxide (N2O5) decomposes to form nitrogen dioxide (NO2) and oxygen (O2). This reaction is first order. The balanced equation is 2N2O5(g)4NO2(g)+O2(g) Use
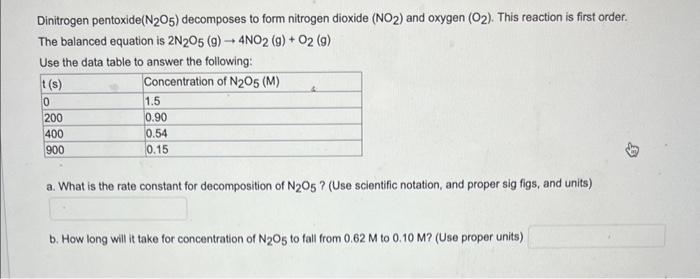
Dinitrogen pentoxide (N2O5) decomposes to form nitrogen dioxide (NO2) and oxygen (O2). This reaction is first order. The balanced equation is 2N2O5(g)4NO2(g)+O2(g) Use the data table to answer the following: a. What is the rate constant for decomposition of N2O5 ? (Use scientific notation, and proper sig figs, and units) b. How long will it take for concentration of N2O5 to fall from 0.62M to 0.10M ? (Use proper units)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


