Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please solve these questions a, b, c with explaination. The work should be in hand writing. Thank you so much. I will thumb up to
Please solve these questions a, b, c with explaination. The work should be in hand writing. Thank you so much. I will thumb up to your work as soon as possible when I receive the answer.
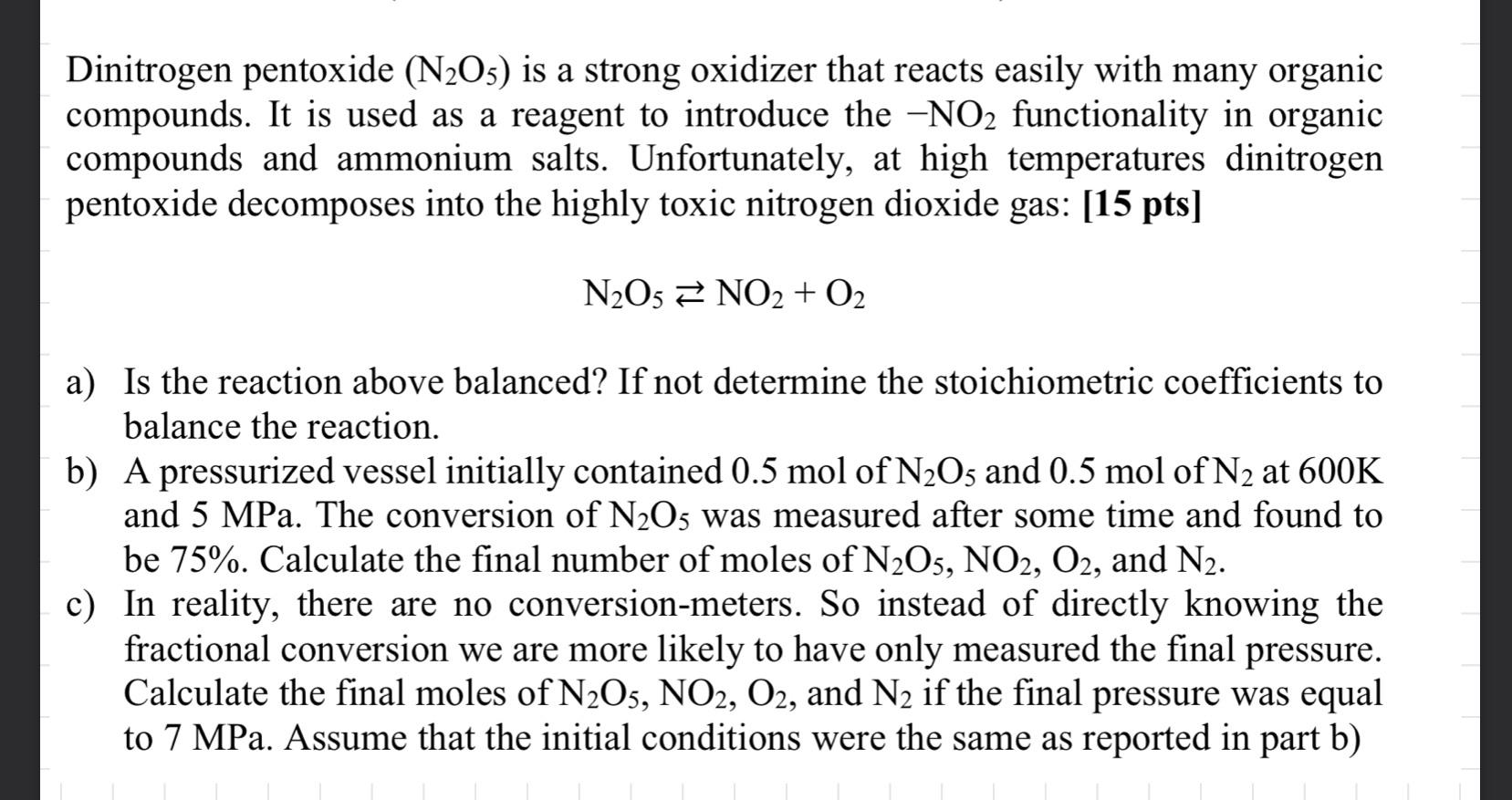
Dinitrogen pentoxide (N2O5) is a strong oxidizer that reacts easily with many organic compounds. It is used as a reagent to introduce the -NO functionality in organic compounds and ammonium salts. Unfortunately, at high temperatures dinitrogen pentoxide decomposes into the highly toxic nitrogen dioxide gas: [15 pts] N2O5 NO2 + O2 a) Is the reaction above balanced? If not determine the stoichiometric coefficients to balance the reaction. b) A pressurized vessel initially contained 0.5 mol of N2O5 and 0.5 mol of N2 at 600K and 5 MPa. The conversion of N2O5 was measured after some time and found to be 75%. Calculate the final number of moles of N2O5, NO2, O2, and N2. c) In reality, there are no conversion-meters. So instead of directly knowing the fractional conversion we are more likely to have only measured the final pressure. Calculate the final moles of N2O5, NO2, O2, and N2 if the final pressure was equal to 7 MPa. Assume that the initial conditions were the same as reported in part b)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


