Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help fill in the missing data, show your working. This is an old quiz I'm trying to understand where I went wrong. Thanks The
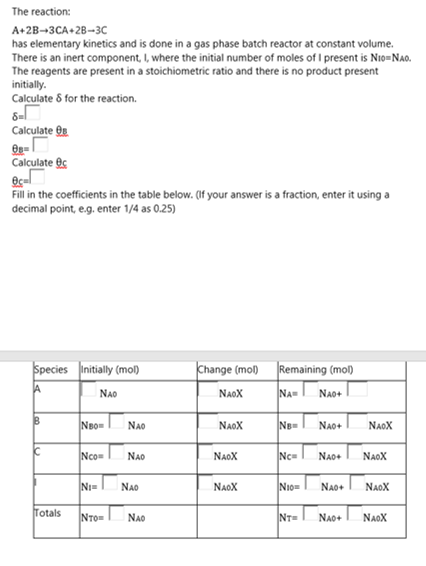
Please help fill in the missing data, show your working. This is an old quiz I'm trying to understand where I went wrong. Thanks
The reaction: A+2B-3CA+28-30 has elementary kinetics and is done in a gas phase batch reactor at constant volume. There is an inert component where the initial number of moles of I present is Nio=NAO. The reagents are present in a stoichiometric ratio and there is no product present initially. Calculated for the reaction. 8-1 Calculate Os- Calculate es Ocal Fill in the coefficients in the table below. (If your answer is a fraction, enter it using a decimal point, eg, enter 1/4 as 0.25) Species initially (mol) Change (mol) Remaining (mol) NAOX NAR NAO+ NAD B NBON NAO NAOX NB- NAO+ NAOX Ic Ncom NAO NAOX Nc NAO NAOX NE NAD NAOX Now NAO NAOX Totals NTOR NAO NT- NAO+ NAOXStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


