Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help filling in missing data. Solution Number Volume of 0.10 M Volume of 0.10 M NaCl. mL KCI. mL [NaCl] [KCI) 1 100.0 0.0
please help filling in missing data. 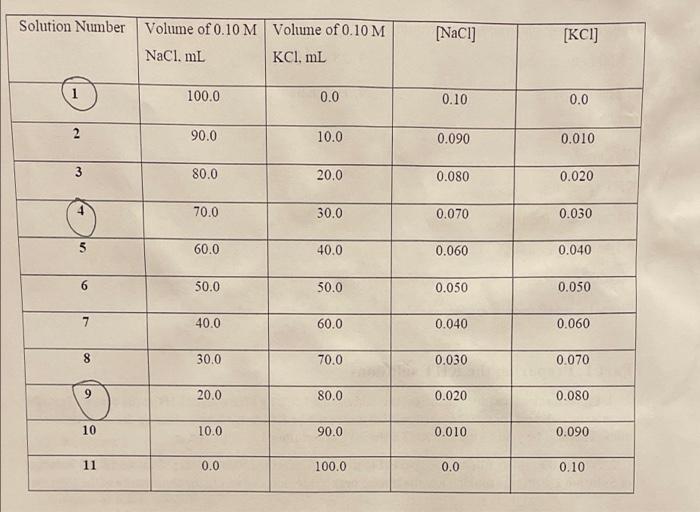
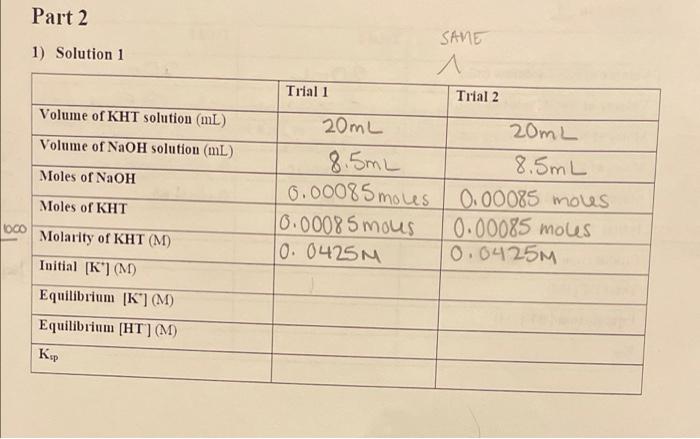
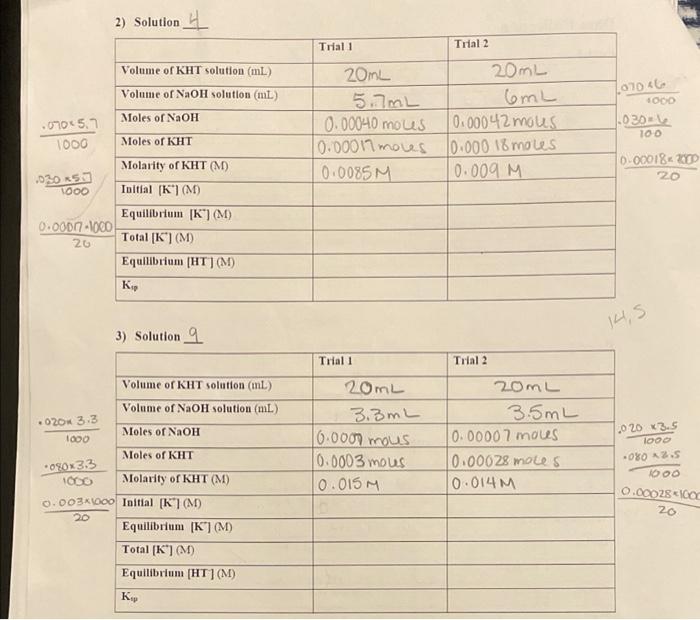
Solution Number Volume of 0.10 M Volume of 0.10 M NaCl. mL KCI. mL [NaCl] [KCI) 1 100.0 0.0 0.10 0.0 2 90.0 10.0 0.090 0.010 3 80.0 20.0 0.080 0.020 70.0 30.0 0.070 0.030 60.0 40.0 0.060 0.040 6 50.0 50.0 0.050 0.050 7 40.0 60.0 0.040 0.060 8 30.0 70.0 0.030 0.070 20.0 80.0 0.020 0.080 10 10.0 90.0 0.010 0.090 11 0.0 100.0 0.0 0.10 Part 2 1) Solution 1 SANE 1 Trial 1 Trial 2 Volume of KHT solution (mL) Volume of NaOH solution (mL) Moles of NaOH 20mL 20mL 8.5mL 8.5mL 0.00085 moles 0.00085 moves 0.0008 5 mous 0.00085 moles 10. 0425M 0.0425M Moles of KHT OCO Molarity of KHT (M) Initial [K] (M) Equilibrium [K] (M) Equilibrium [HT] (M) 2) Solution Trial 1 Trial 2 Volume of KHT solution (m) Volume of NaOH solution (ml) Moles of NaOH 10706 20mL 5.7mL 0.00040 mous 0.00017 moves 0.0085M 07045.7 1000 20 ml 6mL 0.00042 mous 0.000 18 moles 0.009 M 1000 0302 100 Moles of KHT 0.0001820 20 03053 1000 Molarity of KHT (M) Toitial [*](M) Equilibrium [K(M) Total [K](M) Equilibrium [HT](M) 0.00077-1000 26 K, 14,5 3) Solution 9 Trial 1 Trial 2 Volume of KHT solution (m) Volume of NaOH solution (m) 020 3.3 1000 Moles of NaOH 20mL 3.3mL 10.0007 mous 0.0003 mous 10.015M 20mL 3.5mL 0.00007 moues 0.00028 moles 10.014M .020 3.5 1000 080 A 2.5 Moles of KHT *0903.3 1000 0.00028160 20 Molarity of KHT (M) 0.003% 1000 Initial [K"](M) Equilibrium (K) (M) Total [K] (M) Equilibrium [HT](M) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


