Question: Please help!! I am not quite sure that the absorbance is correct and I am super confused as to plotting snd calibrating a curve. A.
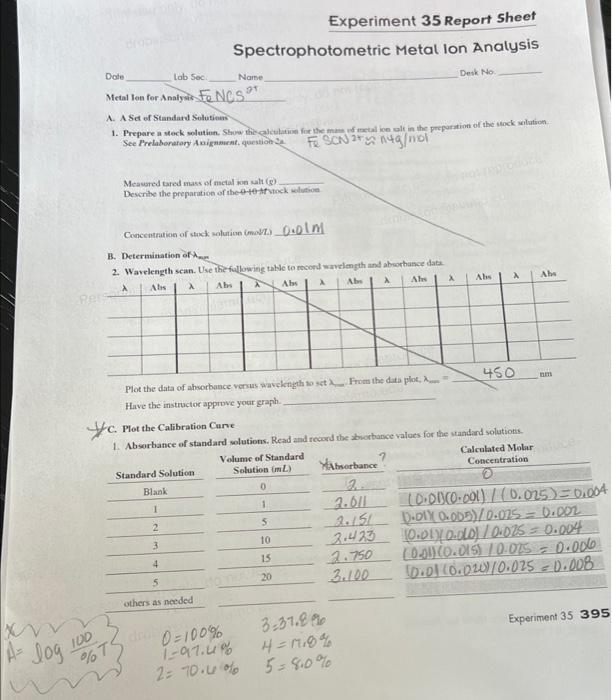

A. A Set of Standard Solutines 1. Prepare a seck solution. Stow the salculation for the man of metal ion alk in the preporation of the siock whation. Mcaused tared mass of metal won alt (g) Describe the preparation of ibe 4+19+t trinek solution Concentration of stack molutise ( most 2.0.0)M B. Deterusination of xme The isidathwine table to rocoed wavelength and abworbunce dats. Plot the data of abworbance venus pavelength so set From the data plot, . . Haye the instructor approve your graph. L. Plot the Calibration Care Calculate the ahsoptivity copfricion for the metal ion. Have the instrector approve your graph.. D. Unknown Metal Ion Coscentration 1. Prepare the unknown. Deactibe the preparation for the solution containing the anknown conceatration of the metal ion. Volume of unknown sample wolation ( mL ) Volume of diluted sample (mL) 2. Concentration of metal ion. Absonbance of metal fos in solution Concentration of metal ion from the calibation curve (mol/L) Concentration of metal ion in the original sample comected for dilution (mol/L) Show your calculations to account for the dilution of the original sample. 3. Expressing Concentration. Express the concentration of the metal ion in the sample in appropriate units of masd volume [i.e., pph (pereent). ppm, ppb, etc.]. Concentration of metal ion in the original sample (masylvolume) Show calculations. Laboratory Questions Circle the questions that have been assigned. 1. Part A.1. For the preparation of the stock solution, 0.01MHNO is ased as a diluent rather than deionized water. Explain why. Hint: Review the solubility rules of transition metal ions in Appendix E. 2. Part A.2. In diluting the standard solutions, 0.01MHNO, is used, ln the dilution, is it more important to ese the correct volume or the correct concentration of the HNO solution for the dilution? Explain. 3. Part B.1. Calibration of the spectrophotometer to set the max is important for a spectrophotometric analysis. Referring to Figure 35.1, suppose the wavelength for all subsequent absorbance measurements had beea set at 450 nm instead o the mar Explain how this would affect the recorded absorbance values for subsequent test solutions. Note: It is ne because the absorbance values would be wrong! 4. Part B.2. Will Fe' Fe3+(aq) or Cu2+(aq) have the shorter mat for absorption in the visible spectrum? Explain. Htr Fe3+(aq) has an orange-brown color, and Cu2+(aq) has a blue color. See Dry Lab 3. 5. Part D.I. An electrolytic-bath sample containing the metal ion of unknown concentration contains suspended matt Because of a lack of time, the abstrbance of the sample was measured and recorded as is. As a result, will the cono tration of the metal jon in the unknown be reported as too high. too low, or remain unchanged as a result of chems hasty decision? Explain. 6. Part D.2. The cuvet used for the absortance measurments is not wiped clean with a Kimwipe before the absorbe measurement. As a result, will the concentration of the metal ion in the unknown be reported as too high, too lov remain unchanged as a result of this poor technique? Explain. A. A Set of Standard Solutines 1. Prepare a seck solution. Stow the salculation for the man of metal ion alk in the preporation of the siock whation. Mcaused tared mass of metal won alt (g) Describe the preparation of ibe 4+19+t trinek solution Concentration of stack molutise ( most 2.0.0)M B. Deterusination of xme The isidathwine table to rocoed wavelength and abworbunce dats. Plot the data of abworbance venus pavelength so set From the data plot, . . Haye the instructor approve your graph. L. Plot the Calibration Care Calculate the ahsoptivity copfricion for the metal ion. Have the instrector approve your graph.. D. Unknown Metal Ion Coscentration 1. Prepare the unknown. Deactibe the preparation for the solution containing the anknown conceatration of the metal ion. Volume of unknown sample wolation ( mL ) Volume of diluted sample (mL) 2. Concentration of metal ion. Absonbance of metal fos in solution Concentration of metal ion from the calibation curve (mol/L) Concentration of metal ion in the original sample comected for dilution (mol/L) Show your calculations to account for the dilution of the original sample. 3. Expressing Concentration. Express the concentration of the metal ion in the sample in appropriate units of masd volume [i.e., pph (pereent). ppm, ppb, etc.]. Concentration of metal ion in the original sample (masylvolume) Show calculations. Laboratory Questions Circle the questions that have been assigned. 1. Part A.1. For the preparation of the stock solution, 0.01MHNO is ased as a diluent rather than deionized water. Explain why. Hint: Review the solubility rules of transition metal ions in Appendix E. 2. Part A.2. In diluting the standard solutions, 0.01MHNO, is used, ln the dilution, is it more important to ese the correct volume or the correct concentration of the HNO solution for the dilution? Explain. 3. Part B.1. Calibration of the spectrophotometer to set the max is important for a spectrophotometric analysis. Referring to Figure 35.1, suppose the wavelength for all subsequent absorbance measurements had beea set at 450 nm instead o the mar Explain how this would affect the recorded absorbance values for subsequent test solutions. Note: It is ne because the absorbance values would be wrong! 4. Part B.2. Will Fe' Fe3+(aq) or Cu2+(aq) have the shorter mat for absorption in the visible spectrum? Explain. Htr Fe3+(aq) has an orange-brown color, and Cu2+(aq) has a blue color. See Dry Lab 3. 5. Part D.I. An electrolytic-bath sample containing the metal ion of unknown concentration contains suspended matt Because of a lack of time, the abstrbance of the sample was measured and recorded as is. As a result, will the cono tration of the metal jon in the unknown be reported as too high. too low, or remain unchanged as a result of chems hasty decision? Explain. 6. Part D.2. The cuvet used for the absortance measurments is not wiped clean with a Kimwipe before the absorbe measurement. As a result, will the concentration of the metal ion in the unknown be reported as too high, too lov remain unchanged as a result of this poor technique? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


