Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help, i need all of what the bottom paragraph is asking for, this is all the information that was given. Citric acid is one
please help, i need all of what the bottom paragraph is asking for, this is all the information that was given. 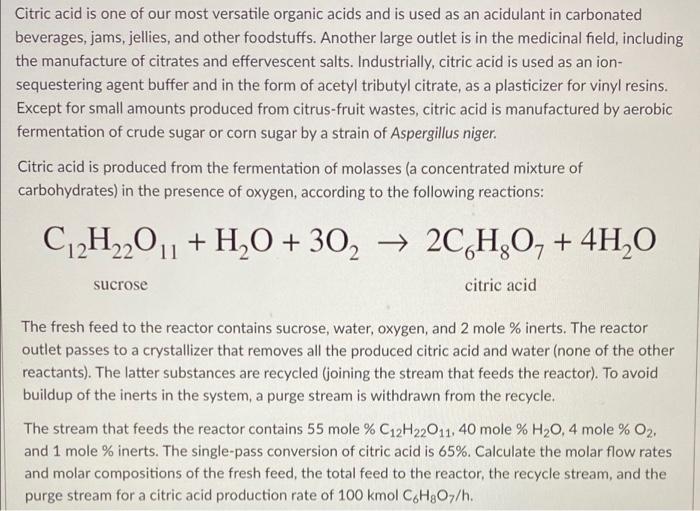
Citric acid is one of our most versatile organic acids and is used as an acidulant in carbonated beverages, jams, jellies, and other foodstuffs. Another large outlet is in the medicinal field, including the manufacture of citrates and effervescent salts. Industrially, citric acid is used as an ion- sequestering agent buffer and in the form of acetyl tributyl citrate, as a plasticizer for vinyl resins. Except for small amounts produced from citrus-fruit wastes, citric acid is manufactured by aerobic fermentation of crude sugar or corn sugar by a strain of Aspergillus niger. Citric acid is produced from the fermentation of molasses (a concentrated mixture of carbohydrates) in the presence of oxygen, according to the following reactions: C12H2201 + H2O + 302 2CH,O, + 4H20 sucrose citric acid The fresh feed to the reactor contains sucrose, water, oxygen, and 2 mole % inerts. The reactor outlet passes to a crystallizer that removes all the produced citric acid and water (none of the other reactants). The latter substances are recycled (joining the stream that feeds the reactor). To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The stream that feeds the reactor contains 55 mole % C12H22011, 40 mole % H20,4 mole % O2, and 1 mole % inerts. The single-pass conversion of citric acid is 65%. Calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream, and the purge stream for a citric acid production rate of 100 kmol CH307/h 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


