Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help. im not sure how to do this... thankyou the question is the first picture The average bond enthalpy for CH is 413kJmol1,413kJmol1 of
please help. im not sure how to do this... thankyou the question is the first picture 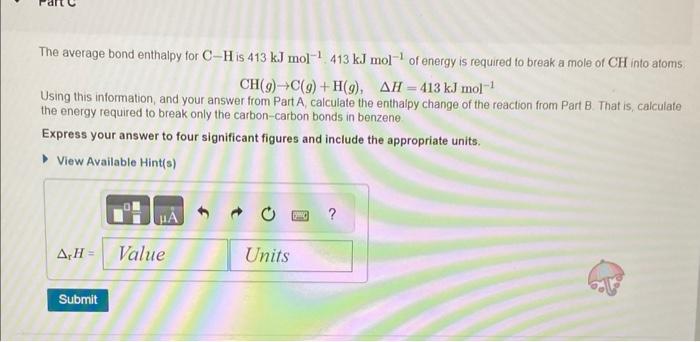
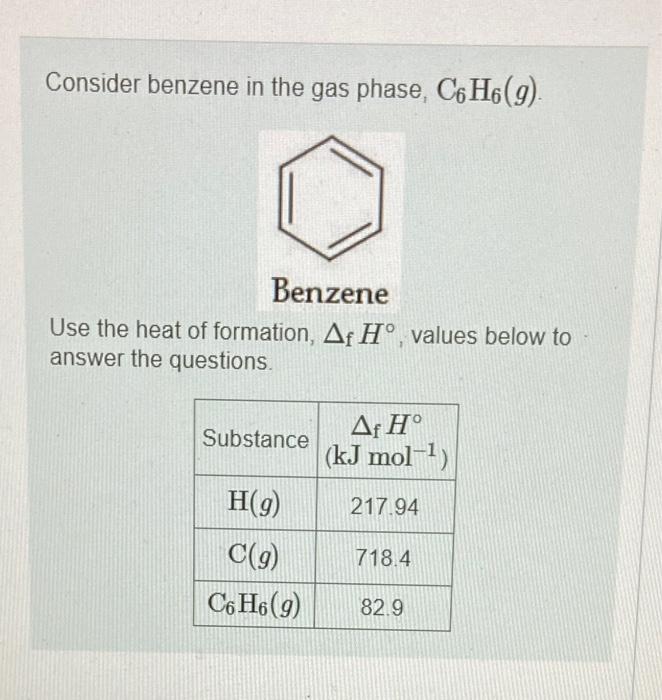
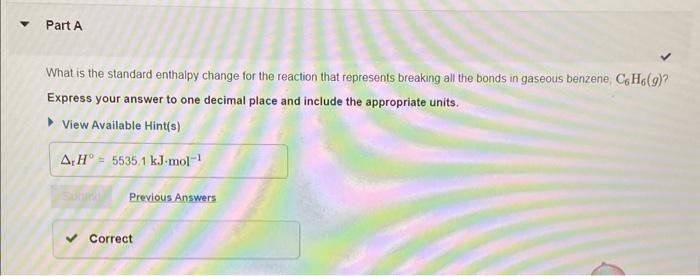
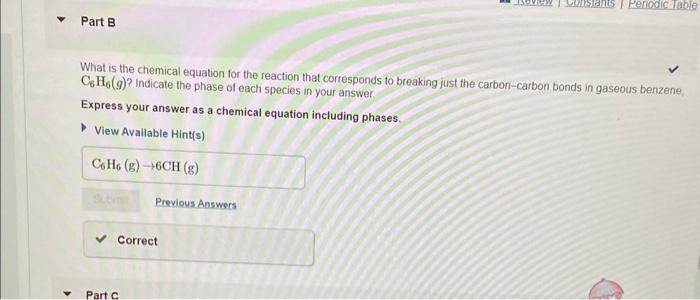
The average bond enthalpy for CH is 413kJmol1,413kJmol1 of energy is required to break a mole of CH into atoms. CH(g)C(g)+H(g),H=413kJmol1 Using this information, and your answer from Part A, calculate the enthalpy change of the reaction from Part B. That is, calculate the energy required to break only the carbon-carbon bonds in benzene Express your answer to four significant figures and include the appropriate units. Consider benzene in the gas phase, C6H6(g). Use the heat of formation, fH, values below to answer the questions. What is the standard enthalpy change for the reaction that represents breaking all the bonds in gaseous benzene, C6H6(g) ? Express your answer to one decimal place and include the appropriate units. View Available Hintis) What is the chemical equation for the reaction that corresponds to breaking just the carbon-carbon bonds in gaseous benzene, C6H6(g) ? Indicate the phase of each species in your answer. Express your answer as a chemical equation including phases. View Avaliable Hint(s) 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


