Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help! less than 10 hours left! thank you! 4. Calculate the change in energy for each transition, nk=3,4,5,6 and nc=2. Show your work and
please help! less than 10 hours left! thank you! 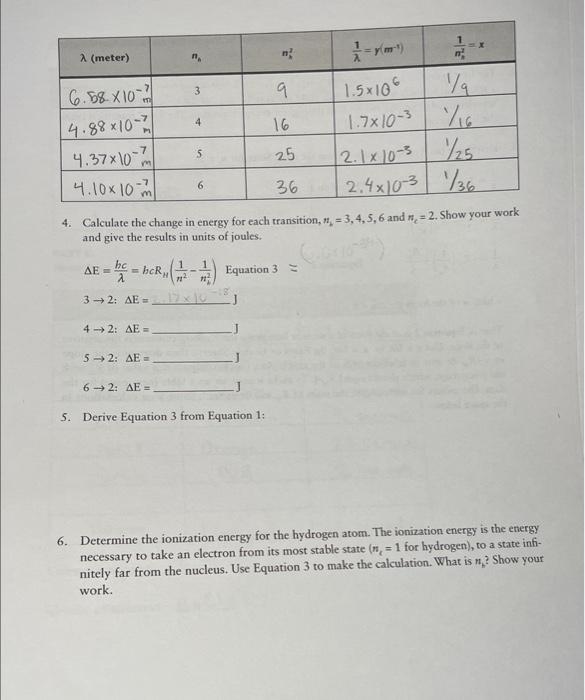
4. Calculate the change in energy for each transition, nk=3,4,5,6 and nc=2. Show your work and give the results in units of joules. E=bc=bcRH(n21n21)Equation3=32:E=3JJ42:E=52:E=62:E= 5. Derive Equation 3 from Equation 1: 6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy necessary to take an electron from its most stable state ( nc=1 for hydrogen), to a state infinitely far from the nucleus. Use Equation 3 to make the calculation. What is nh ? Show your work. 4. Calculate the change in energy for each transition, nk=3,4,5,6 and nc=2. Show your work and give the results in units of joules. E=bc=bcRH(n21n21)Equation3=32:E=3JJ42:E=52:E=62:E= 5. Derive Equation 3 from Equation 1: 6. Determine the ionization energy for the hydrogen atom. The ionization energy is the energy necessary to take an electron from its most stable state ( nc=1 for hydrogen), to a state infinitely far from the nucleus. Use Equation 3 to make the calculation. What is nh ? Show your work 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


