Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me answer all parts of this question. Thank you! When it says refer to 2C this is what is meant. Please help with
Please help me answer all parts of this question. Thank you! 

When it says "refer to 2C" this is what is meant. Please help with #9! 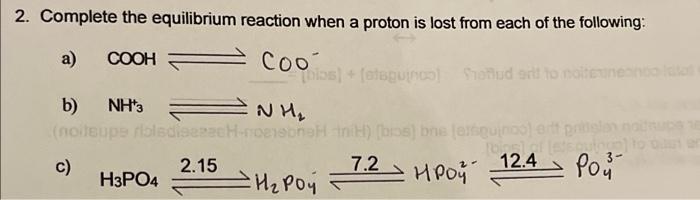
9. Make 100 mL of a 300 mM sodium phosphate buffer at pH 6.5. a) A sodium phosphate buffer will contain phosphates with different levels of pronation depending on the desired pH. Refer to 20 and write the acid/base reaction that will yield a buffer at pH 6.5 (always write the acidic form on the left). b) Calculate the mass of reagents required to prepare the following stocks:100 ml of a 400 mm monosodium phosphate solution (FW 137.99g/mol), and 100 ml of a 600 mM disodium phosphate (FW 174.20g/mol) solution 7 CHE343 Buffer WS c) You have created 400 mm monophosphate stock and 600 mm disodium phosphate stock. Which method will you need to use to create your final phosphate buffer, the mix method or the conversion method? d) What is the total concentration of the buffer? (conjugate] + (acid] => e) Use the Henderson-Hasselbalch equation to write a second relationship between the (conjugate) and facid). Solve for the ratio of (conjugate] to (acid). 1) Calculate the volume of each of these solutions that will be mixed to make 100 mL of a 300 mm sodium phosphate buffer pH 6.5. Don't forget to calculate the amount of water to add 2. Complete the equilibrium reaction when a proton is lost from each of the following: a) COOH Cool L) (oleguinto) Sonuda oma b) ) NH'S (noleupe naladie NH eenHH) (bos) belebunla 7.2 Por H Poy HRoy c) 2.15 12.4 H3PO4 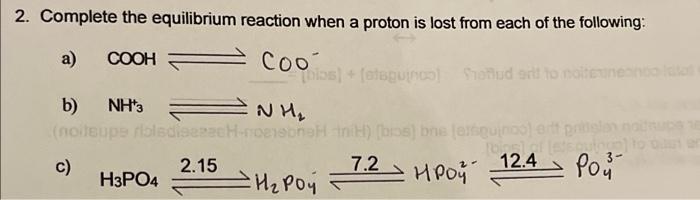
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


