Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me answer at least one if possible! Use the References to access important values if needed for this question. The vapor pressure of
Please help me answer at least one if possible! 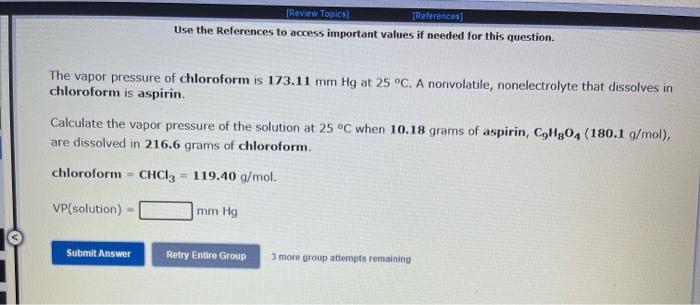
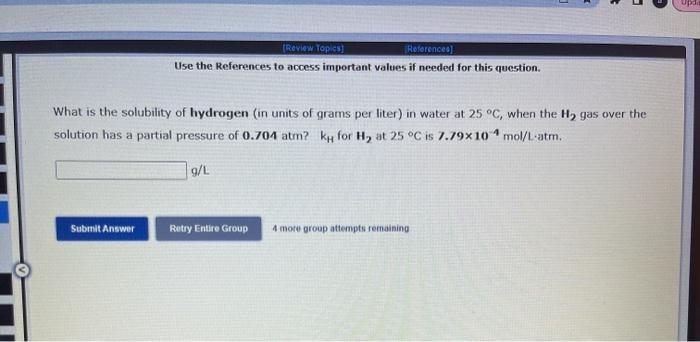
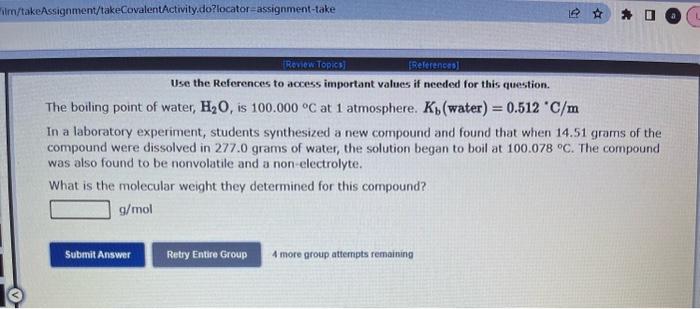
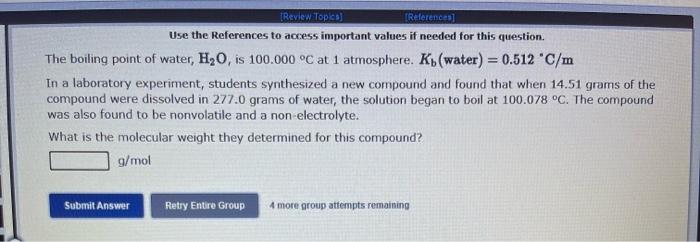
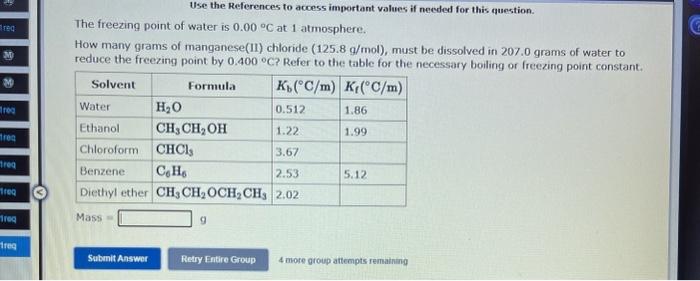
Use the References to access important values if needed for this question. The vapor pressure of chloroform is 173.11mmHg at 25C. A nonvolatile, nonelectrolyte that dissolves in chloroform is aspirin. Calculate the vapor pressure of the solution at 25C when 10.18grams of aspirin, C9H8O4(180.1g/mol, are dissolved in 216.6 grams of chloroform. chloroform =CHCl3=119.40g/mol VP( solution )=mmHg What is the solubility of hydrogen (in units of grams per liter) in water at 25C, when the H2 gas over the solution has a partial pressure of 0.704 atm? kH for H2 at 25C is 7.79104mol/Latm. Use the References to access important values if needed for this question. The boiling point of water, H2O, is 100.000C at 1 atmosphere. Kb (water) =0.512C/m In a laboratory experiment, students synthesized a new compound and found that when 14.51grams of the compound were dissolved in 277.0 grams of water, the solution began to boil at 100.078C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? gf mol Use the References to access important values if needed for this question. The boiling point of water, H2O, is 100.000C at 1 atmosphere. Kb (water) =0.512C/m In a laboratory experiment, students synthesized a new compound and found that when 14.51 grams of the compound were dissolved in 277.0 grams of water, the solution began to boil at 100.078C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol 4 more group attempts remaining Use the References to access important values if needed for this question. The freezing point of water is 0.00C at 1 atmosphere. How many grams of manganese(II) chloride (125.8g/mol), must be dissolved in 207.0grams of water to reduce the freezing point by 0.400C ? Refer to the table for the necessary boiling or freezing point constant 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


