Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me answer the questions some of them i got partially right and im not aure which one. Nitrogen normally has 7 protons and
please help me answer the questions some of them i got partially right and im not aure which one. 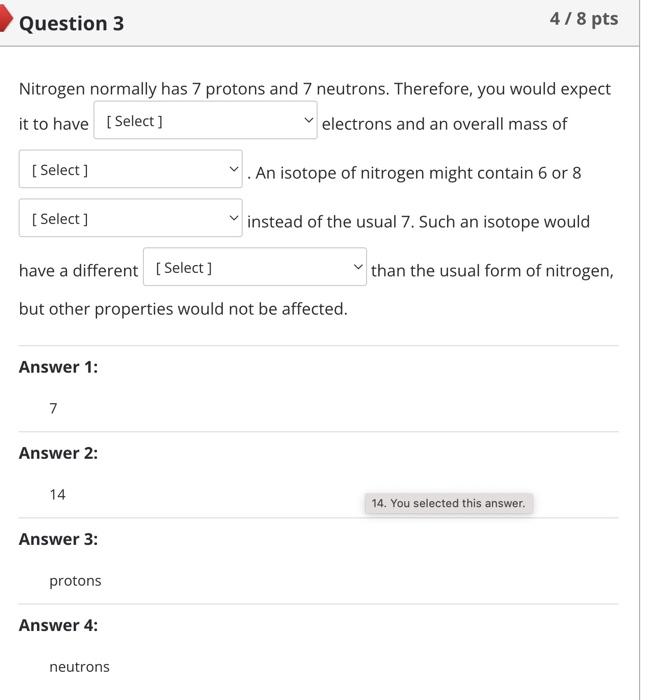
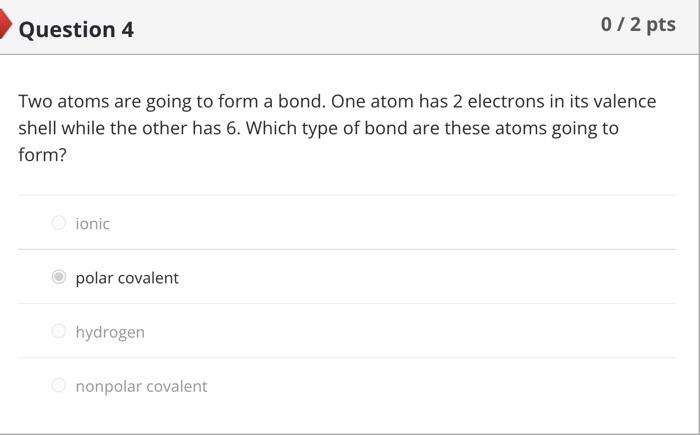

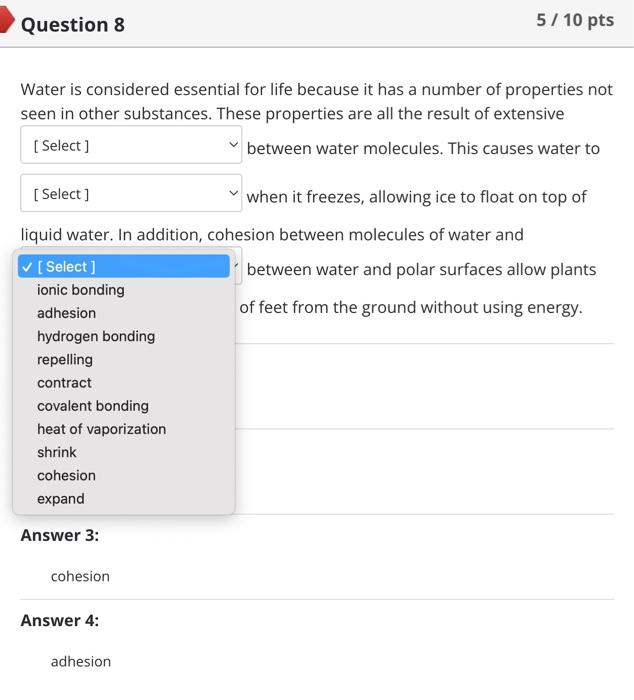
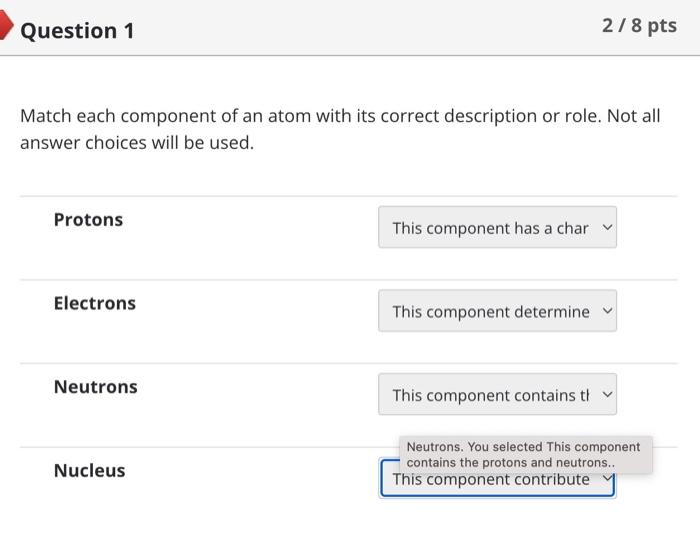
Nitrogen normally has 7 protons and 7 neutrons. Therefore, you would expect it to have electrons and an overall mass of . An isotope of nitrogen might contain 6 or 8 instead of the usual 7. Such an isotope would have a different than the usual form of nitrogen, but other properties would not be affected. Two atoms are going to form a bond. One atom has 2 electrons in its valence shell while the other has 6 . Which type of bond are these atoms going to form? ionic polar covalent hydrogen nonpolar covalent Covalent bonds formed between hydrogen and either oxygen or nitrogen are the most polar bonds encountered in Biology. The attractions between two molecules that each contain such very polar bonds are called .... oxygenitrogen interactions hydrogen bonds ionic bonds nonpolar covalent bonds Question 8 5/10pts Water is considered essential for life because it has a number of properties not seen in other substances. These properties are all the result of extensive [ Select ] between water molecules. This causes water to [Select] when it freezes, allowing ice to float on top of liquid water. In addition, cohesion between molecules of water and \begin{tabular}{l|l} \hline [ Select ] & between water and polar surfaces allow plants \\ ionic bonding adhesion & of feet from the ground without using energy. \\ hydrogen bonding & \\ repelling & \\ contract & \\ covalent bonding \\ heat of vaporization \\ shrink \\ cohesion \\ expand \end{tabular} Answer 3: cohesion Answer 4: adhesion Match each component of an atom with its correct description or role. Not all answer choices will be used. Protons Electrons Neutrons Nucleus 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


