Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE help me answer these. super lost Calculate the specific heat capacity of a metal if a 17.0g sample requires 481J to change the temperature
PLEASE help me answer these. super lost 

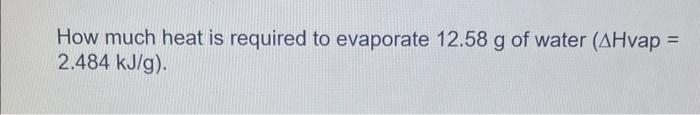

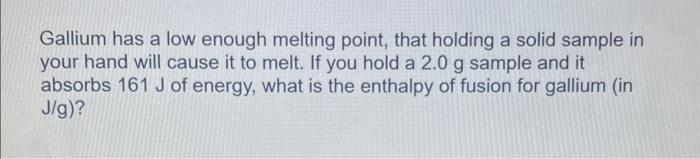

Calculate the specific heat capacity of a metal if a 17.0g sample requires 481J to change the temperature of the metal from 25.0C to 67.0C. 5.0g of copper was heated from 20.0C to 80.0C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.384 J/gC) How much heat is required to evaporate 12.58g of water (Hvap= 2.484kJ/g) A candle made of parafin wax was melted. It absorbed 90.8kJ of heat. If the enthalpy of fusion is 0.200kJ/g, what was the mass of the candle? Gallium has a low enough melting point, that holding a solid sample in your hand will cause it to melt. If you hold a 2.0g sample and it absorbs 161J of energy, what is the enthalpy of fusion for gallium (in J/g )? How many kJ of energy does it take to change 36.0g of ice at 15.0 C to water at 0.C ? The specific heat of ice is 2.10J/gC and the heat of fusion ( Hfus) for ice is 334.0J/g 





Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


