Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE HELP ME ANSWER THIS QUESTION ASAP b. Volumetric analysis measures the volume of a solution of known concentration (titrant) that is needed to determine
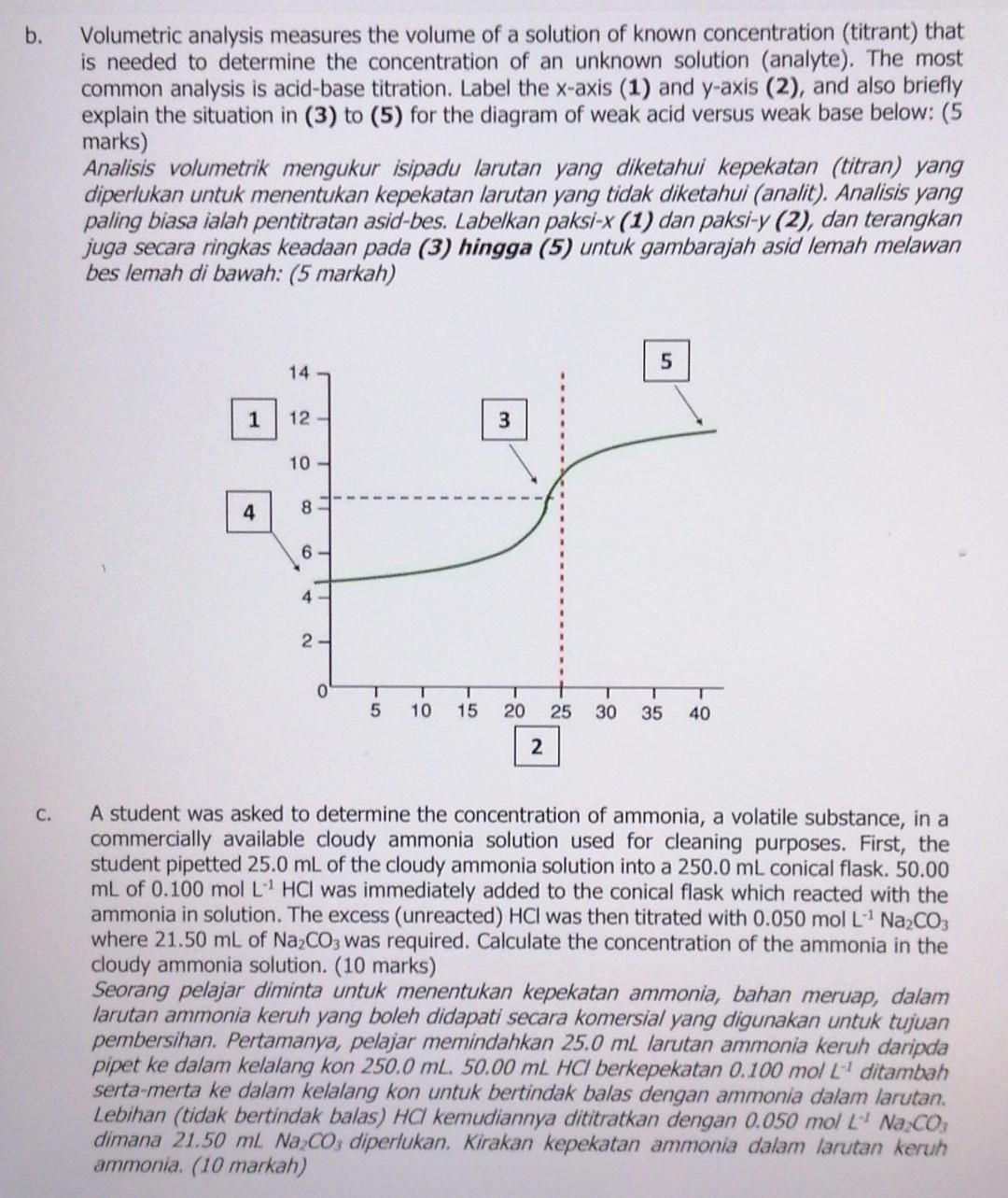
PLEASE HELP ME ANSWER THIS QUESTION ASAP
b. Volumetric analysis measures the volume of a solution of known concentration (titrant) that is needed to determine the concentration of an unknown solution (analyte). The most common analysis is acid-base titration. Label the x-axis (1) and y-axis (2), and also briefly explain the situation in (3) to (5) for the diagram of weak acid versus weak base below: (5 marks) Analisis volumetrik mengukur isipadu larutan yang diketahui kepekatan (titran) yang diperlukan untuk menentukan kepekatan larutan yang tidak diketahui (analit). Analisis yang paling biasa ialah pentitratan asid-bes. Labelkan paksi-X (1) dan paksi-y (2), dan terangkan juga secara ringkas keadaan pada (3) hingga (5) untuk gambarajah asid lemah melawan bes lemah di bawah: (5 markah) 5 14 1 12 3 10 4 8 6 4 1 1 2. - T 5 T 10 15 20 25 30 T 35 T 40 2 C. A student was asked to determine the concentration of ammonia, a volatile substance, in a commercially available cloudy ammonia solution used for cleaning purposes. First, the student pipetted 25.0 mL of the cloudy ammonia solution into a 250.0 mL conical flask. 50.00 mL of 0.100 mol L HCI was immediately added to the conical flask which reacted with the ammonia in solution. The excess (unreacted) HCI was then titrated with 0.050 mol L" Na2CO3 where 21.50 mL of Na2CO3 was required. Calculate the concentration of the ammonia in the cloudy ammonia solution. (10 marks) Seorang pelajar diminta untuk menentukan kepekatan ammonia, bahan meruap, dalam larutan ammonia keruh yang boleh didapati secara komersial yang digunakan untuk tujuan pembersihan. Pertamanya, pelajar memindahkan 25.0 mL larutan ammonia keruh daripda pipet ke dalam kelalang kon 250.0 ml. 50.00 ml HCI berkepekatan 0.100 mol L ditambah serta-merta ke dalam kelalang kon untuk bertindak balas dengan ammonia dalam larutan. Lebihan (tidak bertindak balas) HC kemudiannya dititratkan dengan 0.050 mol L Na Co, dimana 21.50 ml Na Co; diperlukan. Kirakan kepekatan ammonia dalam larutan keruh ammonia. (10 markah)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


