Question: please help me answering number 3(a,b,c,d,e) Experiment 4 Fractional Crystallization Theory: Fractional crystallization is a technique used to separate components of a mixture relying on
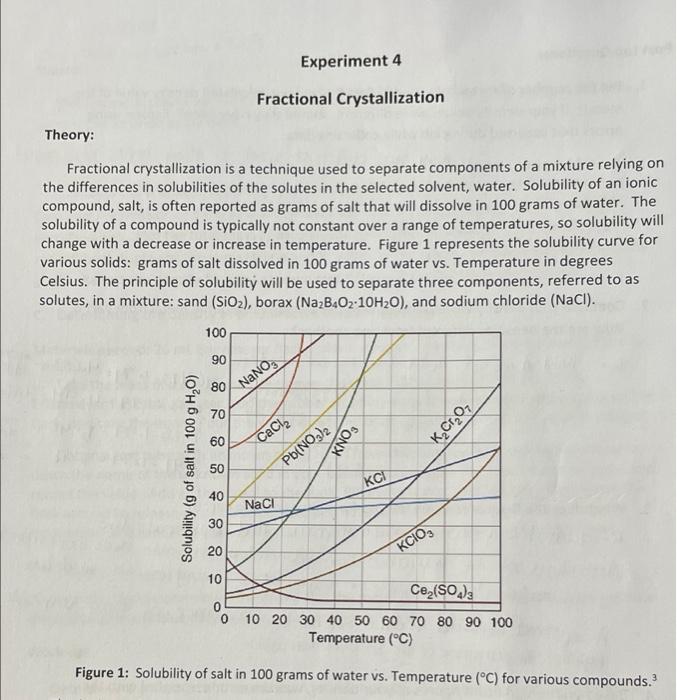
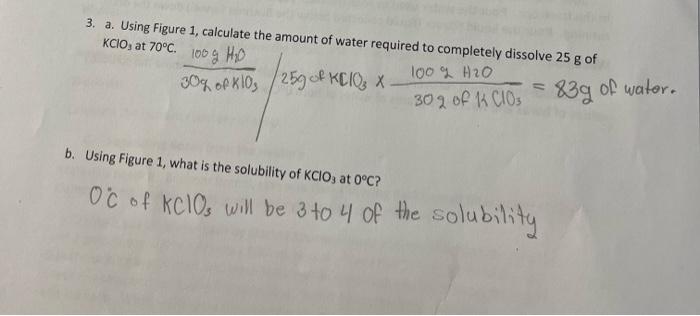

Experiment 4 Fractional Crystallization Theory: Fractional crystallization is a technique used to separate components of a mixture relying on the differences in solubilities of the solutes in the selected solvent, water. Solubility of an ionic compound, salt, is often reported as grams of salt that will dissolve in 100 grams of water. The solubility of a compound is typically not constant over a range of temperatures, so solubility will change with a decrease or increase in temperature. Figure 1 represents the solubility curve for various solids: grams of salt dissolved in 100 grams of water vs. Temperature in degrees Celsius. The principle of solubility will be used to separate three components, referred to as solutes, in a mixture: sand (SiO2), borax (Na2B4O2-10H20), and sodium chloride (NaCl). 100 90 o 80 NaNO3 70 60 KCrO7 CaCl2 SONY Solubility (g of salt in 100 g H,0) 50 Pb(NO3)2 KCI 40 NaCl 30 20 KCIO, 10 0 0 Ce (SO4)3 10 20 30 40 50 60 70 80 90 100 Temperature (C) Figure 1: Solubility of salt in 100 grams of water vs. Temperature (C) for various compounds. 3. a. Using Figure 1, calculate the amount of water required to completely dissolve 25 g of KCIO, at 70C 100 g HD 30g of klos / 25g of KClO3 x. 100% H2O A 302 of K CIOs - 839 of water. b. Using Figure 1, what is the solubility of KClO3 at 0C? Oc of KClOs will be 3 to 4 of the solubility C. Using your answers from parts 3a and 3b, what amount of KCIO: originally added would remain in solution once cooled to 0C? d. What amount of KCIO, originally added in question 3a should precipitate out of the solution once cooled to 0C. e. If the student recovered 15 grams of KClO3 at the end of the experiment, what percentage of KCIO; was recovered
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


