Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me by solving this example, i want to know how it works. thank u Consider the liquid reaction below: 1) Desrired: A2Br1=k1CA2 Endothermic
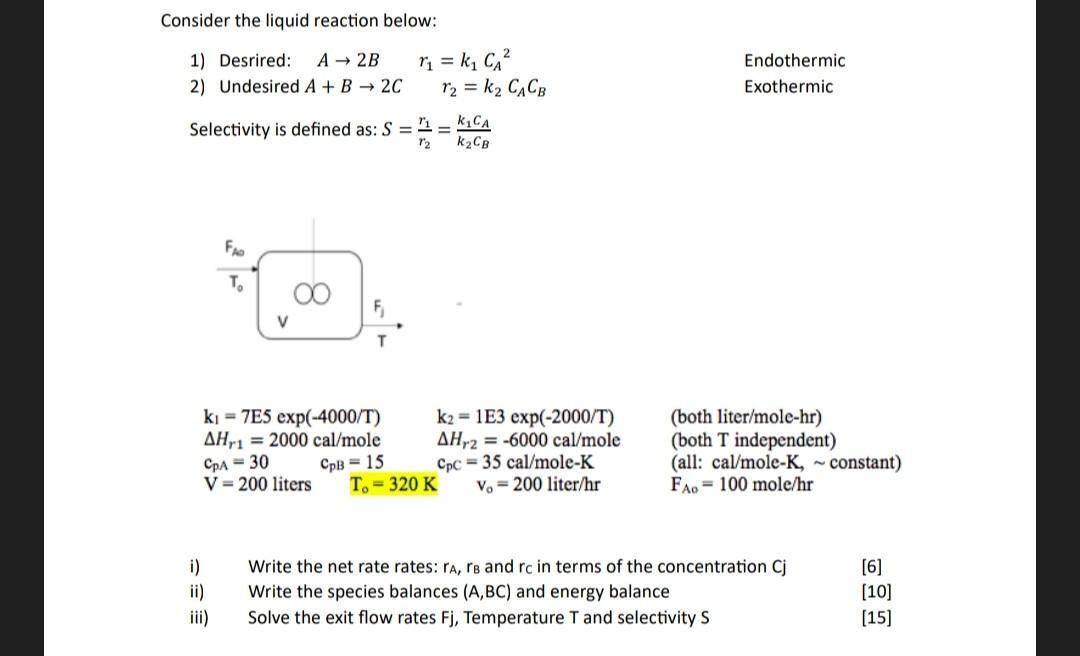
Please help me by solving this example, i want to know how it works. thank u
Consider the liquid reaction below: 1) Desrired: A2Br1=k1CA2 Endothermic 2) Undesired A+B2Cr2=k2CACB Exothermic Selectivity is defined as: S=r2r1=k2CBk1CA k1=7E5exp(4000/T)Hr1=2000cal/molecpA=30cpB=15V=200litersk2=1E3exp(2000/T)Hr2=6000cal/molecpC=35cal/moleKTo=320Kvo=200liter/hr(bothliter/mole-hr)(bothTindependent)(all:cal/moleK,constant)FAo=100mole/hr i) Write the net rate rates: rA,rB and rc in terms of the concentration Cj [6] ii) Write the species balances (A,BC) and energy balance [10] iii) Solve the exit flow rates Fj, Temperature T and selectivity S [15]Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


