Question: Please help me do these Q, and my R = 0, please substitute R = 0 (Q2 and Q3) to do, and make sure that
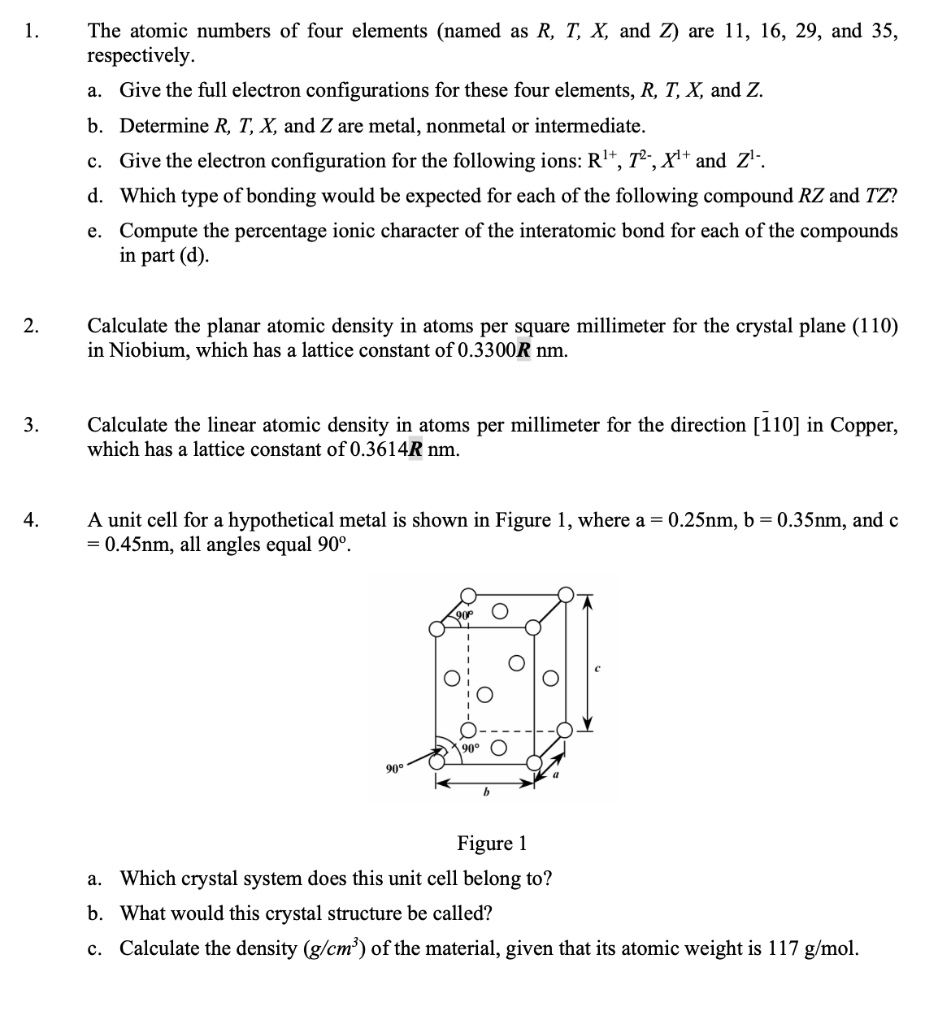
Please help me do these Q, and my R = 0, please substitute R = 0 (Q2 and Q3) to do, and make sure that the answers should be correct (with specific steps). Thanks!
1. The atomic numbers of four elements (named as R, T, X, and Z) are 11, 16, 29, and 35, respectively. a. Give the full electron configurations for these four elements, R, T, X, and Z. b. Determine R, T, X, and Z are metal, nonmetal or intermediate. c. Give the electron configuration for the following ions: Rl+, 72-, X1+ and z':. d. Which type of bonding would be expected for each of the following compound RZ and TZ? e. Compute the percentage ionic character of the interatomic bond for each of the compounds in part (d). 2. Calculate the planar atomic density in atoms per square millimeter for the crystal plane (110) in Niobium, which has a lattice constant of 0.3300R nm. 3. Calculate the linear atomic density in atoms per millimeter for the direction [110] in Copper, which has a lattice constant of 0.3614R nm. 4. A unit cell for a hypothetical metal is shown in Figure 1, where a = 0.25nm, b=0.35nm, and c = 0.45nm, all angles equal 90. 90 Figure 1 a. Which crystal system does this unit cell belong to? b. What would this crystal structure be called? c. Calculate the density (g/cm) of the material, given that its atomic weight is 117 g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


