please help me fill in the blanks
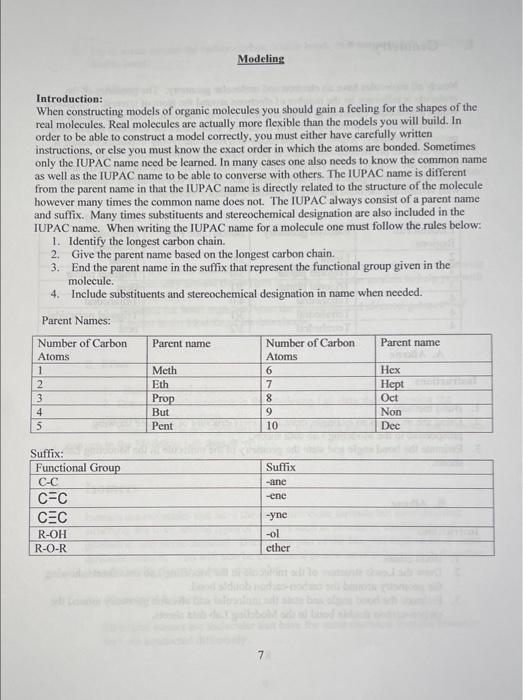
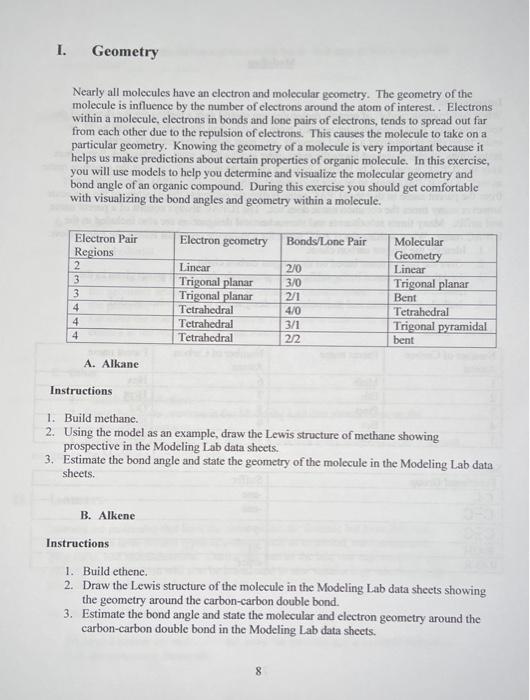
Introduction: When constructing models of organic molecules you should gain a feeling for the shapes of the real molecules. Real molecules are actually more flexible than the models you will build. In order to be able to construct a model correctly, you must either have carefully written instructions, or else you must know the exact order in which the atoms are bonded. Sometimes only the IUPAC name need be learned. In many cases one also needs to know the common name as well as the IUPAC name to be able to converse with others. The IUPAC name is different from the parent name in that the IUPAC name is directly related to the structure of the molecule however many times the common name does not. The IUPAC always consist of a parent name and suffix. Many times substituents and stereochemical designation are also included in the IUPAC name. When writing the IUPAC name for a molecule one must follow the rules below: 1. Identify the longest carbon chain. 2. Give the parent name based on the longest carbon chain. 3. End the parent name in the suffix that represent the functional group given in the molecule. 4. Include substituents and stereochemical designation in name when needed. Parent Names: I. Geometry Nearly all molecules have an clectron and molecular geometry. The geometry of the molecule is influence by the number of electrons around the atom of interest. . Electrons within a molecule, electrons in bonds and lone pairs of electrons, tends to spread out far from each other due to the repulsion of electrons. This causes the molecule to take on a particular geometry. Knowing the geometry of a molecule is very important because it helps us make predictions about certain properties of organic molecule. In this exercise, you will use models to help you determine and visualize the molecular geometry and bond angle of an organic compound. During this exercise you should get comfortable with visualizing the bond angles and geometry within a molecule. A. Alkane Instructions 1. Build methane. 2. Using the model as an example, draw the Lewis structure of methane showing prospective in the Modeling Lab data sheets. 3. Estimate the bond angle and state the geometry of the molecule in the Modeling Lab data sheets. B. Alkene Instructions 1. Build ethene. 2. Draw the Lewis structure of the molecule in the Modeling Lab data sheets showing the geometry around the carbon-carbon double bond. 3. Estimate the bond angle and state the molecular and electron geometry around the carbon-carbon double bond in the Modeling Lab data sheets. 4. Try to rotate around the carbon-carbon double bond. Is it easy to rotate around the double bond? C. Alkyne Instruction 1. Build ethyne. 2. Draw the Lewis structure of ethyne in the Modeling Lab data sheets showing the geometry around the carbon-carbon triple bond. 3. Estimate the bond angle and state the molecular and electron geometry around the carbon-carbon triple bond on the Modeling Lab data sheets. D. Alcohol 1. Build ethanol. 2. Draw the Lewis structure of ethanol in the Modeling Lab data sheets showing the geometry around the oxygen in ethanol. Use the models to help you with the geometry. 3. Estimate the bond angle and state the molecular and electron geometry of ethanol in the Modeling Lab data sheets. E. Ether 1. Build dipropyl ether. 2. Draw the Lewis structure in the Modeling Lab data sheets showing the geometry around oxygen. 3. Estimate the bond angle and state the molecular and electron geometry around oxygen in the Modeling Lab data sheets. II. Isomers Isomers are molecules that have the same molecular formula but have different structure. We have several types of isomers such as structural, conformational, geometric, and stereochemical. During this part of the lab, you explore all of these different isomers. You will also be introduced to new terminology which describes the relationship between these molecules. A. Structural Isomers Structural isomer are molecules that have the same chemical formula however they are connected differently. D. Stereochemistry Stereoisomers are molecules that have the same molecular formula and connectivity but atoms are orientated differently in space. Stereoisomers can be enantiomers or diastereomers of each other depending on whether or not they are mirror images of each other. Enantiomers are nonsuperimposible mirror images of each other and diastereomers are not. We differentiate the two isomer be assigning each isomer stereochemistry designations: cis and trans, E/Z, and R and S. This part of the experiment will introduce you to stereochemistry. When determining the type of isomer, sometimes you will have to determine the priority of your substitutents. Priority is assigned based on atomic numbers. Those that have higher priority will be assigned priority number closer to 1. If two groups have the same priority, keep moving out until you find an atom that have a different atomic number. A. Geometric Isomers (Cis-trans isomers) Cis and trans isomers are shown for only cyclic rings and alkenes. Cis means same side. Trans means opposite sides. a. Alkene 1. Build 2-butene. Place the alkyl groups around the double bond on the same side of the double bond. 2. Is this molecule cis or trans? 3. Draw the Skeletal structure of this molecule showing the orientation of the alkyl group around the double bond. 4. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. For example: (stereochemistry) (carbon containing the double bond) (parent name) 5. Keep the structure you originally build. 6. Rebuild 2-butene. Place the alkyl groups around the double bond on opposite side of the double bond. 7. Draw the skeletal structure of this molecule showing the orientation of the alkyl group around the double bond. 8. Is this molecule cis or trans? 9. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. 10. Try to superimpose the two structures. 11. Can the two structure be superimposed? 12. Are they mirror images of each other? 13. These two structure are diastereomers of each other. b. Ring Systems 14. Build a model of cyclohexane. 15. Remove one hydrogen from the ring and replace it with a methyl group. 16. On the fourth carbon away (the carbon with the methyl group should be counted as 1), replace a hydrogen with a fluorine group and place it on the same side of the ring. 17. Is the molecule cis or trans? 18. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. 19. Keep the structure you originally built and rebuild cyclohexane. 20. Remove one hydrogen from the ring and replace it with a methyl group. 21. On the fourth carbon away (the carbon with the fluoro group should be counted as 1), replace a hydrogen atom with a fluorine group and place it on the opposite side of the ring. 22. Draw the skeletal structure of the molecule and include stereochemistry. Keep in mind the geometry of each atom in the molecule. 23. Try to superimpose the two structures. 24. Is this molecule cis or trans? 25. Name this compound in the Modeling Lab Data Sheet and include stereochemistry, 26. Can the two structure be superimposed? 27. Are they mirror images of each other? 28. These molecules are diastereomers of cach other. B. E and Z system The E and Z system is used when there is more than two groups around the carbon carbon double bond. Using this system requires you to assign priorities to each group around the carbon-carbon double bond. Priority is assigned based on atomic numbers. The group with the highest priority will be assigned priority number of 1 . If two groups have the same priority, keep moving out until you find an atom that have a different atomic number. E - higher priority groups are on opposite sides of the double bond. Z-higher priority groups are on the same side of the double bond. 29. Build ethene. 30. Remove both hydrogen atoms and place a methyl group and a bromo group on carbon 1. 31. Place an ethyl group on carbon 2. Place it on the same side of the double bond in which you placed the bromo group. 32. Draw the skeletal structure of the molecule and include stereochemistry. Keep in mind the geometry of each atom in the molecule. 33. Does this molecule have an E or Z configuration? 34. Name the compound above. For Example: (stercochenuistry)-(position)-(substituent)-(position)-(lengthandfunctionalgroup) 35. Keep your original structure and repeat step 27 and 28 . 36. Place an ethyl group on carbon 2 on the opposite side of the double bond in which you placed the bromo group. 37. Does this molecule have an E or Z configuration? 38. Try to superimpose the two structures. 39. Can the two structure be superimposed? 40. Are they mirror images of each other? 41. These molecules are diastereomers of each other. C. R and S configuration R and S is a stereochemical designation that we also use to distinguish stereoisomers for which there is a non-superimposable mirror image, called an enantiomer. This characteristic is called chirality. These isomers will have one or more chiral centers. These are usually carbon atoms bonded to four distinct atoms or structures. When determining the configuration we must also assign priorities. We must assign priorities to each group around the chiral center. Priorities are assign the same as in the E/Z system. Point the lowest priority group away from you. Move in direction from 1-3. If you moved in a counterclockwise direction the configuration is S, however if you moved in a clockwise direction the configuration is R. 42. Connect a methyl, ethyl, bromo, and chloro group to a carbon atom. 43. Give each group priority (1-4). 44. Hold group number 4 where it points away from you. 45. Count from the highest priority group (1) to the lower priority group (3). 46. Draw the skeletal structure of your molecule showing perspective in the Modeling Lab Data Sheet. 47. Did you go in a clockwise or counter clockwise direction? 48. Is the configuration R or S ? 49. Name the compound and include stereochemistry. 50. Keep your structure and rebuild the same structure however switch the position of the bromo and chloro group. 51. Draw the skeletal structure of your molecule showing perspective in the Modeling Lab Data Sheet. 52. Is the configuration R or S ? 53. Name the compound and include stereochemistry. Introduction: When constructing models of organic molecules you should gain a feeling for the shapes of the real molecules. Real molecules are actually more flexible than the models you will build. In order to be able to construct a model correctly, you must either have carefully written instructions, or else you must know the exact order in which the atoms are bonded. Sometimes only the IUPAC name need be learned. In many cases one also needs to know the common name as well as the IUPAC name to be able to converse with others. The IUPAC name is different from the parent name in that the IUPAC name is directly related to the structure of the molecule however many times the common name does not. The IUPAC always consist of a parent name and suffix. Many times substituents and stereochemical designation are also included in the IUPAC name. When writing the IUPAC name for a molecule one must follow the rules below: 1. Identify the longest carbon chain. 2. Give the parent name based on the longest carbon chain. 3. End the parent name in the suffix that represent the functional group given in the molecule. 4. Include substituents and stereochemical designation in name when needed. Parent Names: I. Geometry Nearly all molecules have an clectron and molecular geometry. The geometry of the molecule is influence by the number of electrons around the atom of interest. . Electrons within a molecule, electrons in bonds and lone pairs of electrons, tends to spread out far from each other due to the repulsion of electrons. This causes the molecule to take on a particular geometry. Knowing the geometry of a molecule is very important because it helps us make predictions about certain properties of organic molecule. In this exercise, you will use models to help you determine and visualize the molecular geometry and bond angle of an organic compound. During this exercise you should get comfortable with visualizing the bond angles and geometry within a molecule. A. Alkane Instructions 1. Build methane. 2. Using the model as an example, draw the Lewis structure of methane showing prospective in the Modeling Lab data sheets. 3. Estimate the bond angle and state the geometry of the molecule in the Modeling Lab data sheets. B. Alkene Instructions 1. Build ethene. 2. Draw the Lewis structure of the molecule in the Modeling Lab data sheets showing the geometry around the carbon-carbon double bond. 3. Estimate the bond angle and state the molecular and electron geometry around the carbon-carbon double bond in the Modeling Lab data sheets. 4. Try to rotate around the carbon-carbon double bond. Is it easy to rotate around the double bond? C. Alkyne Instruction 1. Build ethyne. 2. Draw the Lewis structure of ethyne in the Modeling Lab data sheets showing the geometry around the carbon-carbon triple bond. 3. Estimate the bond angle and state the molecular and electron geometry around the carbon-carbon triple bond on the Modeling Lab data sheets. D. Alcohol 1. Build ethanol. 2. Draw the Lewis structure of ethanol in the Modeling Lab data sheets showing the geometry around the oxygen in ethanol. Use the models to help you with the geometry. 3. Estimate the bond angle and state the molecular and electron geometry of ethanol in the Modeling Lab data sheets. E. Ether 1. Build dipropyl ether. 2. Draw the Lewis structure in the Modeling Lab data sheets showing the geometry around oxygen. 3. Estimate the bond angle and state the molecular and electron geometry around oxygen in the Modeling Lab data sheets. II. Isomers Isomers are molecules that have the same molecular formula but have different structure. We have several types of isomers such as structural, conformational, geometric, and stereochemical. During this part of the lab, you explore all of these different isomers. You will also be introduced to new terminology which describes the relationship between these molecules. A. Structural Isomers Structural isomer are molecules that have the same chemical formula however they are connected differently. D. Stereochemistry Stereoisomers are molecules that have the same molecular formula and connectivity but atoms are orientated differently in space. Stereoisomers can be enantiomers or diastereomers of each other depending on whether or not they are mirror images of each other. Enantiomers are nonsuperimposible mirror images of each other and diastereomers are not. We differentiate the two isomer be assigning each isomer stereochemistry designations: cis and trans, E/Z, and R and S. This part of the experiment will introduce you to stereochemistry. When determining the type of isomer, sometimes you will have to determine the priority of your substitutents. Priority is assigned based on atomic numbers. Those that have higher priority will be assigned priority number closer to 1. If two groups have the same priority, keep moving out until you find an atom that have a different atomic number. A. Geometric Isomers (Cis-trans isomers) Cis and trans isomers are shown for only cyclic rings and alkenes. Cis means same side. Trans means opposite sides. a. Alkene 1. Build 2-butene. Place the alkyl groups around the double bond on the same side of the double bond. 2. Is this molecule cis or trans? 3. Draw the Skeletal structure of this molecule showing the orientation of the alkyl group around the double bond. 4. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. For example: (stereochemistry) (carbon containing the double bond) (parent name) 5. Keep the structure you originally build. 6. Rebuild 2-butene. Place the alkyl groups around the double bond on opposite side of the double bond. 7. Draw the skeletal structure of this molecule showing the orientation of the alkyl group around the double bond. 8. Is this molecule cis or trans? 9. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. 10. Try to superimpose the two structures. 11. Can the two structure be superimposed? 12. Are they mirror images of each other? 13. These two structure are diastereomers of each other. b. Ring Systems 14. Build a model of cyclohexane. 15. Remove one hydrogen from the ring and replace it with a methyl group. 16. On the fourth carbon away (the carbon with the methyl group should be counted as 1), replace a hydrogen with a fluorine group and place it on the same side of the ring. 17. Is the molecule cis or trans? 18. Name this compound in the Modeling Lab Data Sheet and include stereochemistry. 19. Keep the structure you originally built and rebuild cyclohexane. 20. Remove one hydrogen from the ring and replace it with a methyl group. 21. On the fourth carbon away (the carbon with the fluoro group should be counted as 1), replace a hydrogen atom with a fluorine group and place it on the opposite side of the ring. 22. Draw the skeletal structure of the molecule and include stereochemistry. Keep in mind the geometry of each atom in the molecule. 23. Try to superimpose the two structures. 24. Is this molecule cis or trans? 25. Name this compound in the Modeling Lab Data Sheet and include stereochemistry, 26. Can the two structure be superimposed? 27. Are they mirror images of each other? 28. These molecules are diastereomers of cach other. B. E and Z system The E and Z system is used when there is more than two groups around the carbon carbon double bond. Using this system requires you to assign priorities to each group around the carbon-carbon double bond. Priority is assigned based on atomic numbers. The group with the highest priority will be assigned priority number of 1 . If two groups have the same priority, keep moving out until you find an atom that have a different atomic number. E - higher priority groups are on opposite sides of the double bond. Z-higher priority groups are on the same side of the double bond. 29. Build ethene. 30. Remove both hydrogen atoms and place a methyl group and a bromo group on carbon 1. 31. Place an ethyl group on carbon 2. Place it on the same side of the double bond in which you placed the bromo group. 32. Draw the skeletal structure of the molecule and include stereochemistry. Keep in mind the geometry of each atom in the molecule. 33. Does this molecule have an E or Z configuration? 34. Name the compound above. For Example: (stercochenuistry)-(position)-(substituent)-(position)-(lengthandfunctionalgroup) 35. Keep your original structure and repeat step 27 and 28 . 36. Place an ethyl group on carbon 2 on the opposite side of the double bond in which you placed the bromo group. 37. Does this molecule have an E or Z configuration? 38. Try to superimpose the two structures. 39. Can the two structure be superimposed? 40. Are they mirror images of each other? 41. These molecules are diastereomers of each other. C. R and S configuration R and S is a stereochemical designation that we also use to distinguish stereoisomers for which there is a non-superimposable mirror image, called an enantiomer. This characteristic is called chirality. These isomers will have one or more chiral centers. These are usually carbon atoms bonded to four distinct atoms or structures. When determining the configuration we must also assign priorities. We must assign priorities to each group around the chiral center. Priorities are assign the same as in the E/Z system. Point the lowest priority group away from you. Move in direction from 1-3. If you moved in a counterclockwise direction the configuration is S, however if you moved in a clockwise direction the configuration is R. 42. Connect a methyl, ethyl, bromo, and chloro group to a carbon atom. 43. Give each group priority (1-4). 44. Hold group number 4 where it points away from you. 45. Count from the highest priority group (1) to the lower priority group (3). 46. Draw the skeletal structure of your molecule showing perspective in the Modeling Lab Data Sheet. 47. Did you go in a clockwise or counter clockwise direction? 48. Is the configuration R or S ? 49. Name the compound and include stereochemistry. 50. Keep your structure and rebuild the same structure however switch the position of the bromo and chloro group. 51. Draw the skeletal structure of your molecule showing perspective in the Modeling Lab Data Sheet. 52. Is the configuration R or S ? 53. Name the compound and include stereochemistry