Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me fill out the charts and explain the work for the calculation of the time at which the two lines intersect and the



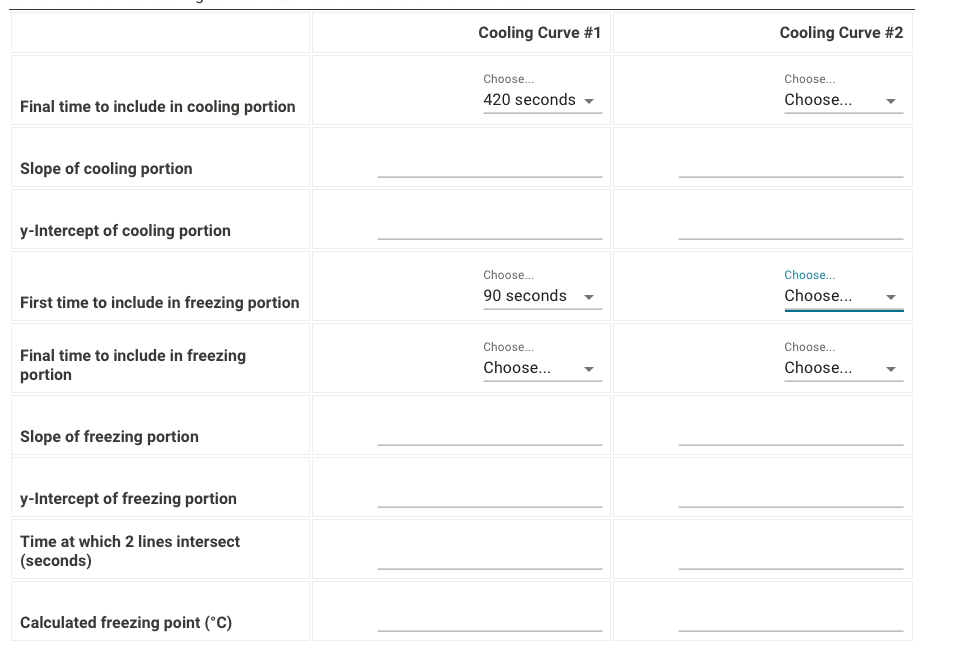


Please help me fill out the charts and explain the work for the calculation of the time at which the two lines intersect and the freezing point for both cooling curves, the molality and moles of unknown in solution, and the molar mass of the unknown, including all units.
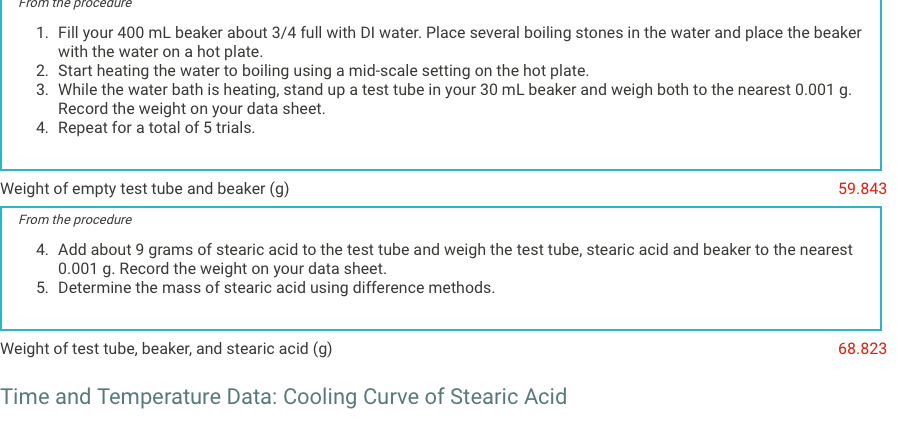
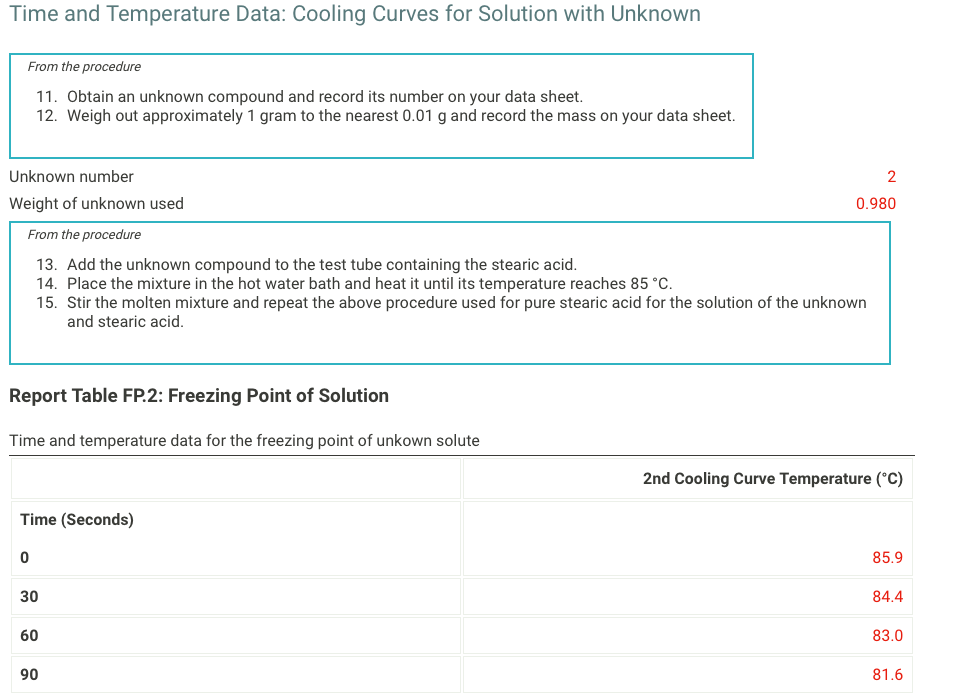
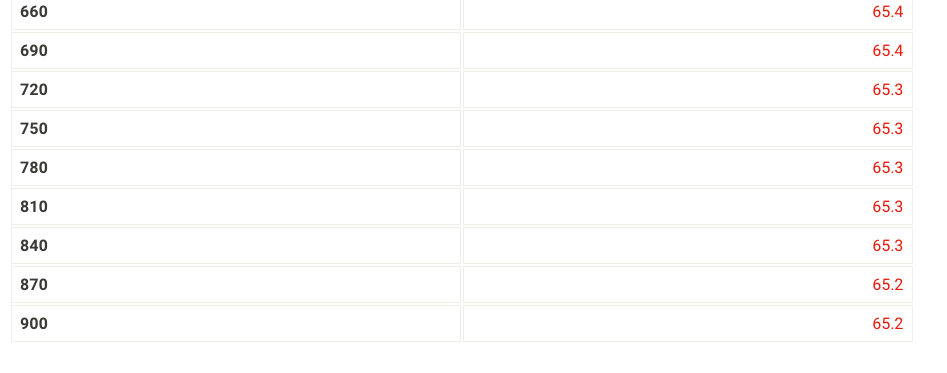
1. Fill your 400mL beaker about 3/4 full with DI water. Place several boiling stones in the water and place the beaker with the water on a hot plate. 2. Start heating the water to boiling using a mid-scale setting on the hot plate. 3. While the water bath is heating, stand up a test tube in your 30mL beaker and weigh both to the nearest 0.001g. Record the weight on your data sheet. 4. Repeat for a total of 5 trials. Neight of empty test tube and beaker (g) 59.84 From the procedure 4. Add about 9 grams of stearic acid to the test tube and weigh the test tube, stearic acid and beaker to the nearest 0.001g. Record the weight on your data sheet. 5. Determine the mass of stearic acid using difference methods. Neight of test tube, beaker, and stearic acid (g) Time and Temperature Data: Cooling Curve of Stearic Acid Report Table FP.1: Freezing Point of Solvent (Stearic Acid) Time and Temperature Data: Cooling Curves for Solution with Unknown From the procedure 11. Obtain an unknown compound and record its number on your data sheet. 12. Weigh out approximately 1gram to the nearest 0.01g and record the mass on your data sheet. Unknown number Weight of unknown used From the procedure 13. Add the unknown compound to the test tube containing the stearic acid. 14. Place the mixture in the hot water bath and heat it until its temperature reaches 85C. 15. Stir the molten mixture and repeat the above procedure used for pure stearic acid for the solution of the unknown and stearic acid. \begin{tabular}{|l|l|l|} \hline 660 & & 65.4 \\ \hline 690 & 65.4 \\ \hline 720 & 65.3 \\ \hline 750 & & \\ \hline 780 & 65.3 \\ \hline 810 & & \\ \hline 840 & & \\ \hline 870 & 65.3 \\ \hline 900 & & \\ \hline \end{tabular} . (5pts) What is most likely the identity of your unknown soluteStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


