Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me find The relative rate of one/two= along with M = for differential analysis part one. And the relative rate of one/relative rate
Please help me 
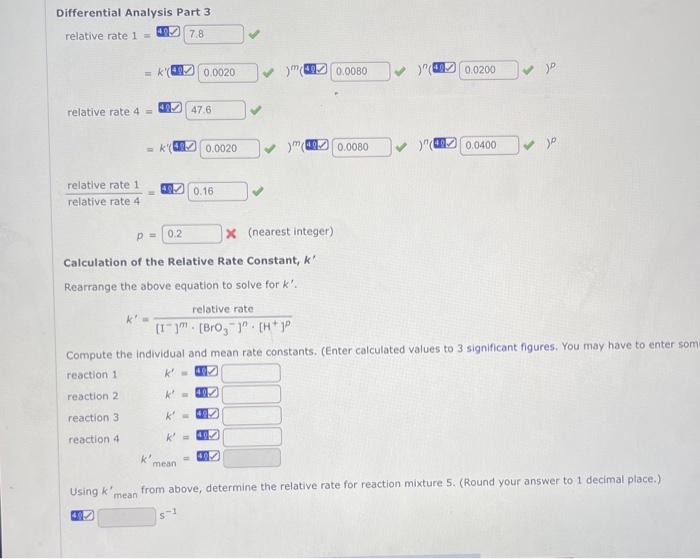
Let m,n, and p stand for the order coefficients in the following equation. relativerate=k[1]m[BrO3]n[H+]p Complete the analyses using the data recorded in the table above. (Enter rate ratios to two decima Differential Analysis Part 1 relative rate 1= relative rate 2= =k(8) relativerate2relativerate1=4IDm= Differential Analysis Part 2 relative rate 1= relative rate 3= Differential Analysis Part 3 relative rate 1= relative rate 4= =k(4) relativerate4relativerate1= p= x (nearest integer) Calculation of the Relative Rate Constant, k Rearrange the above equation to solve for k. k=[I]m[BrO3]n[H+]prelativerate Compute the individual and mean rate constants. (Enter calculated values to 3 significant figures. reaction 1 reaction 2 k=k=0.k=k= reaction3 reaction4k= find The relative rate of one/two= along with M = for differential analysis part one.
And the relative rate of one/relative rate three = along with N = for differential analysis part two
and P= for Differential analysis part three


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


