Answered step by step
Verified Expert Solution
Question
1 Approved Answer
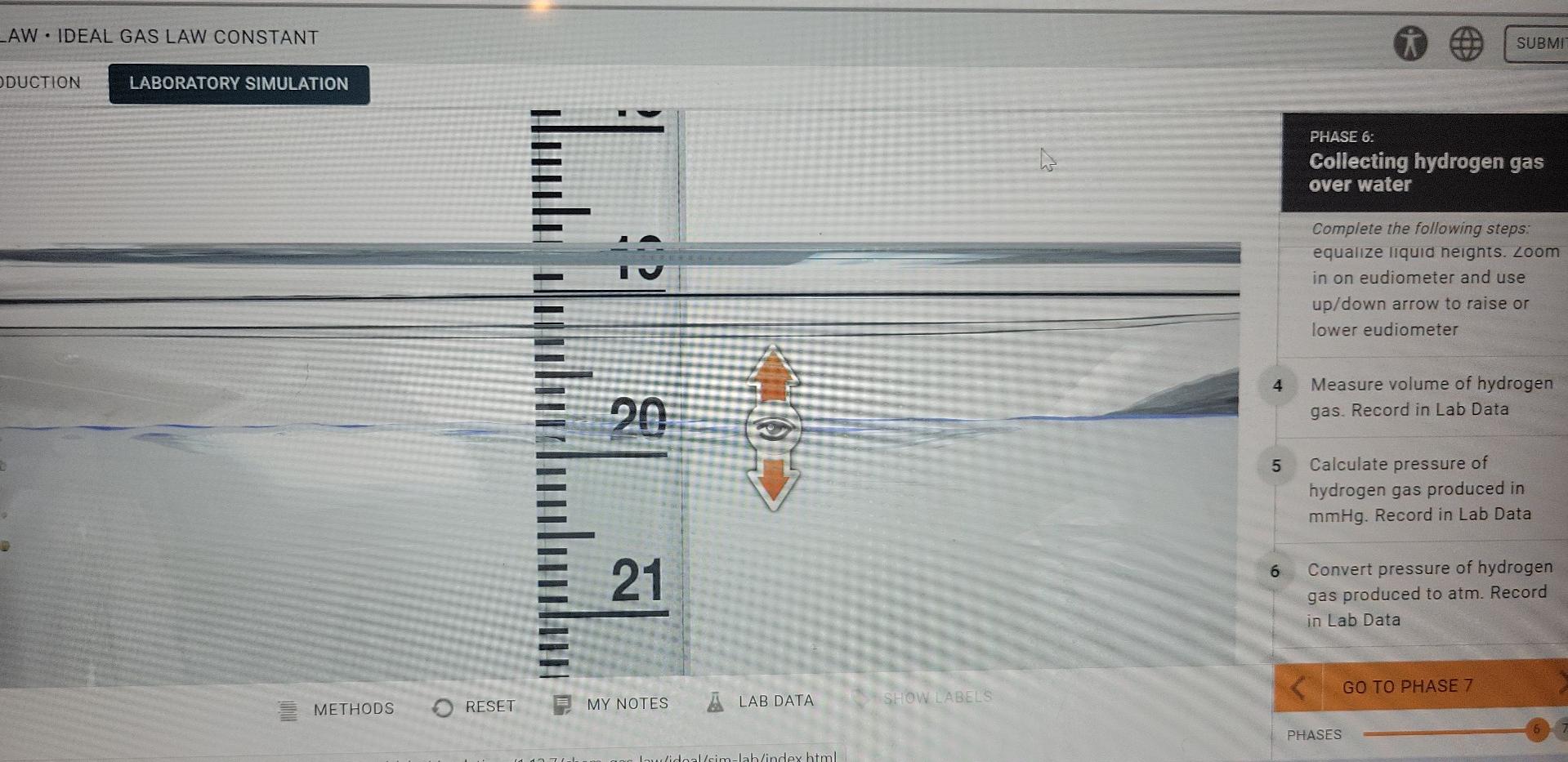
Please help me out and thank you! LAW. IDEAL GAS LAW CONSTANT SUBMI DUCTION LABORATORY SIMULATION ho PHASE 6: Collecting hydrogen gas over water is
Please help me out and thank you!
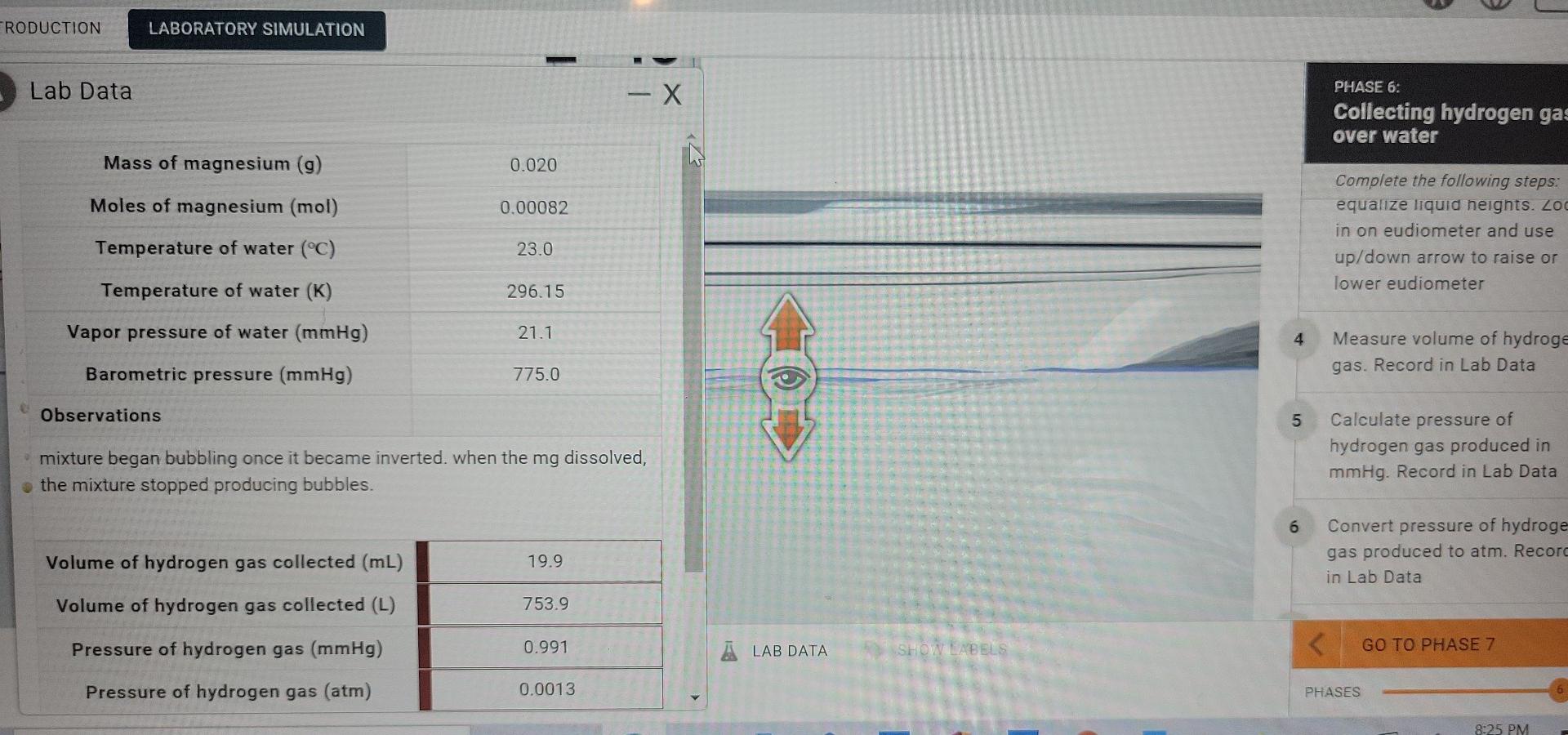
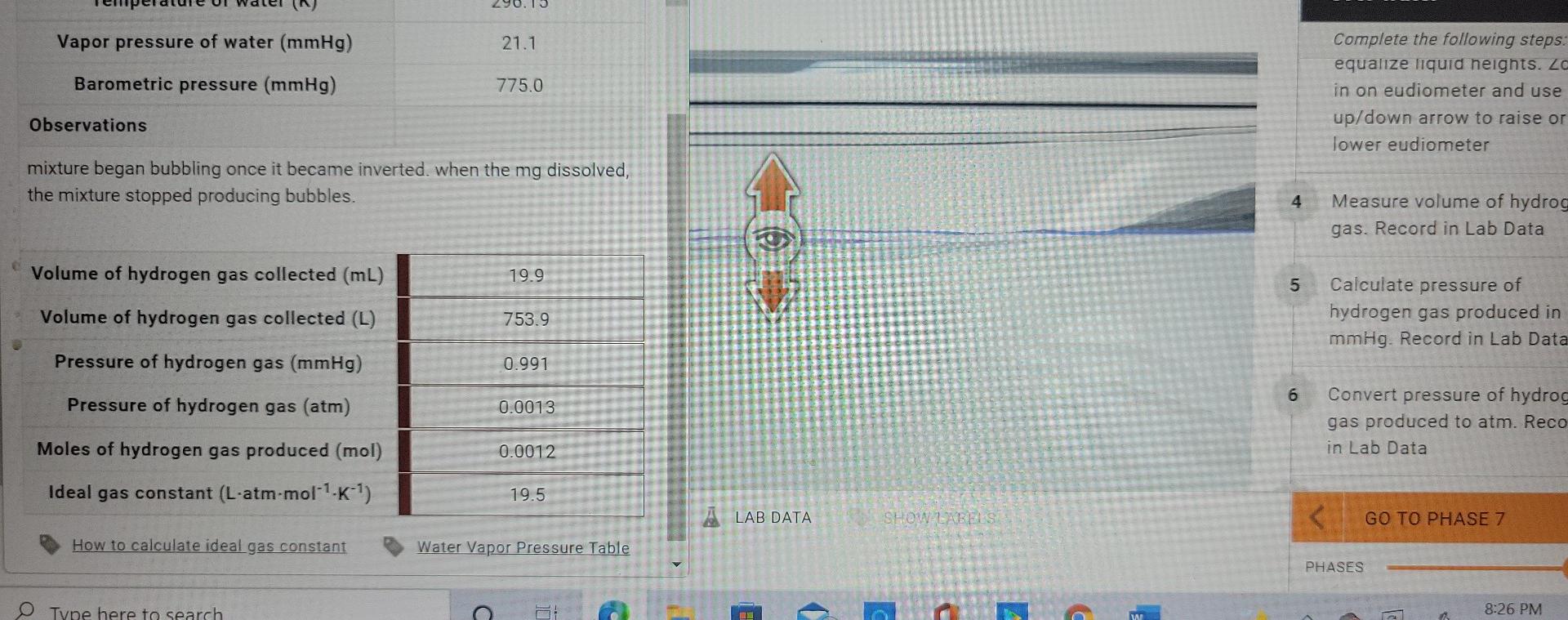
LAW. IDEAL GAS LAW CONSTANT SUBMI DUCTION LABORATORY SIMULATION ho PHASE 6: Collecting hydrogen gas over water is Complete the following steps: equalize liquid heights. Zoom in on eudiometer and use up/down arrow to raise or lower eudiometer 4 20 Measure volume of hydrogen gas. Record in Lab Data 5 Calculate pressure of hydrogen gas produced in mmHg. Record in Lab Data 6 21 Convert pressure of hydrogen gas produced to atm. Record in Lab Data GO TO PHASE 7 METHODS RESET SHOW LABELS MY NOTES A LAB DATA PHASES Iulidoal/sim-lab/indexhtml RODUCTION LABORATORY SIMULATION Lab Data - X PHASE 6: Collecting hydrogen gas over water Mass of magnesium (9) 0.020 Moles of magnesium (mol) 0.00082 Complete the following steps: equalize liquid neights. Zo in on eudiometer and use up/down arrow to raise or lower eudiometer Temperature of water (C) 23.0 Temperature of water (K) 296.15 Vapor pressure of water (mmHg) 21.1 4 Measure volume of hydroge gas. Record in Lab Data Barometric pressure (mmHg) 775.0 Observations 5 Calculate pressure of hydrogen gas produced in mmHg. Record in Lab Data mixture began bubbling once it became inverted. when the mg dissolved, the mixture stopped producing bubbles. 6 Convert pressure of hydroge gas produced to atm. Record in Lab Data Volume of hydrogen gas collected (mL) 19.9 Volume of hydrogen gas collected (L) 753.9 Pressure of hydrogen gas (mmHg) 0.991 LAB DATA SHOW.LABELSStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


