Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me out. thank you so much. The magnesium isotope represented by the nuclide symbol contains protons, neutrons, and electrons. Protons: Neutrons: Eloctrons: 12,
please help me out. thank you so much. 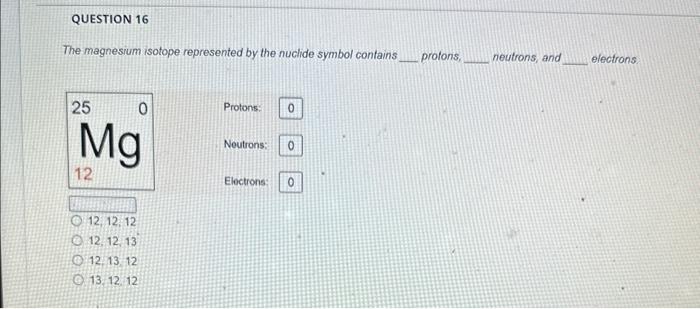
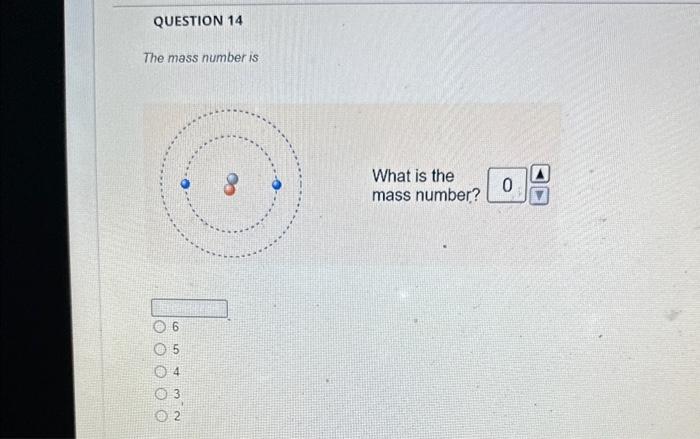
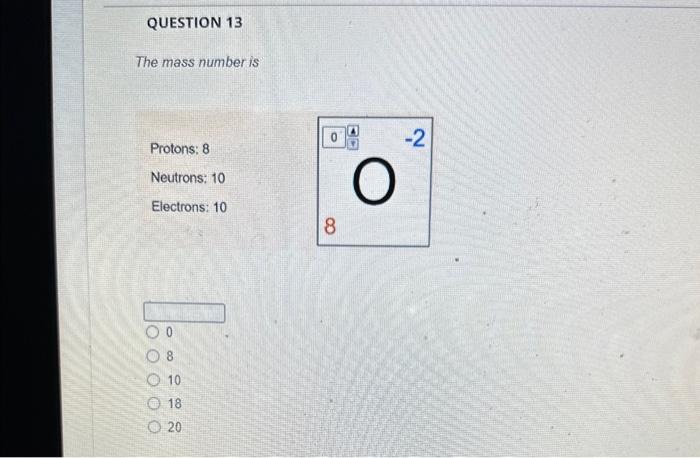
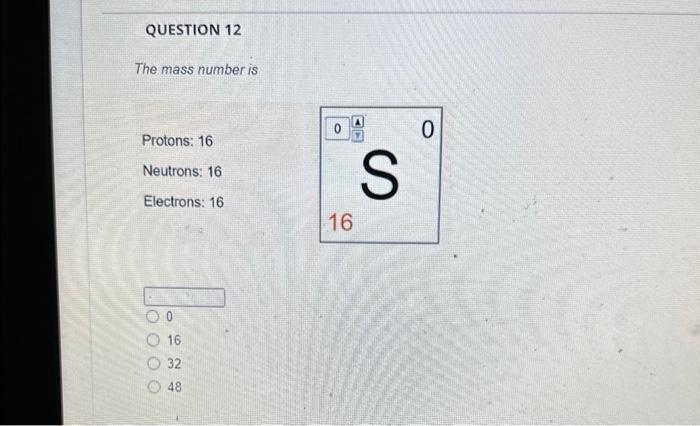
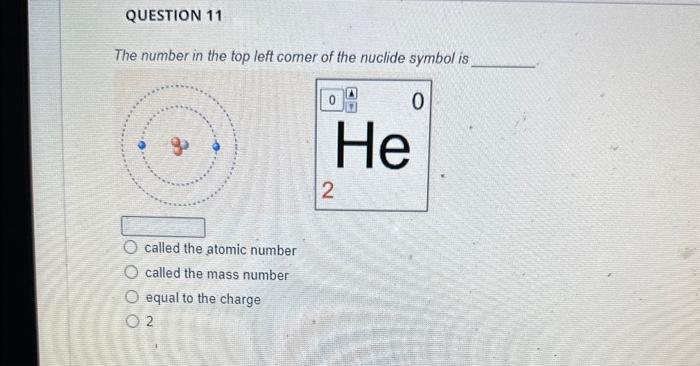
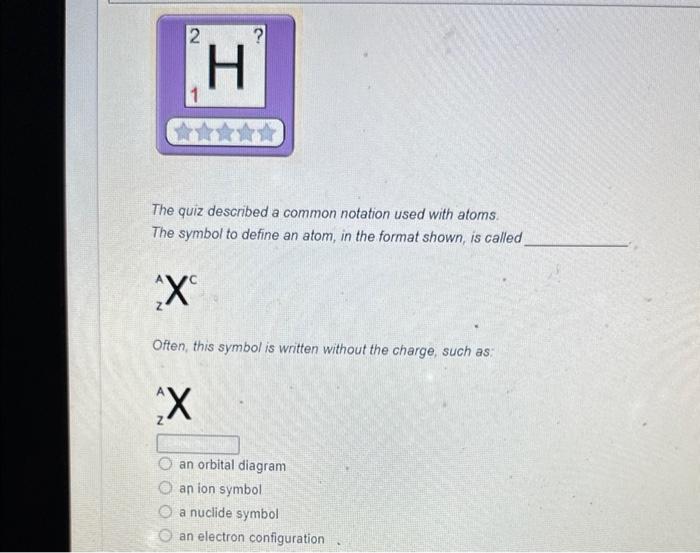
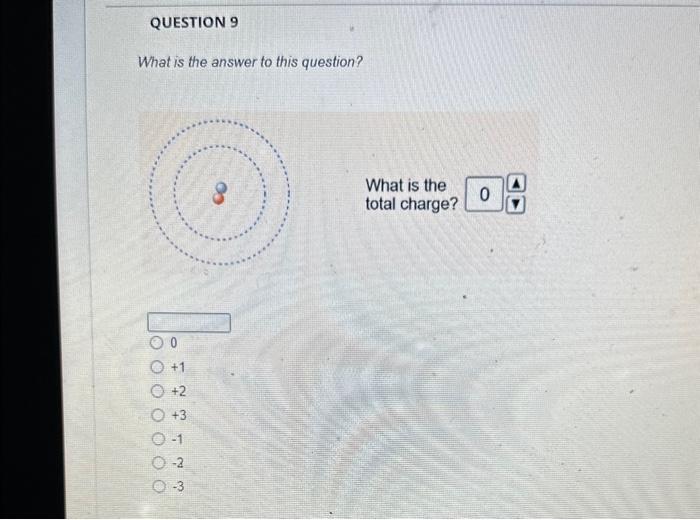
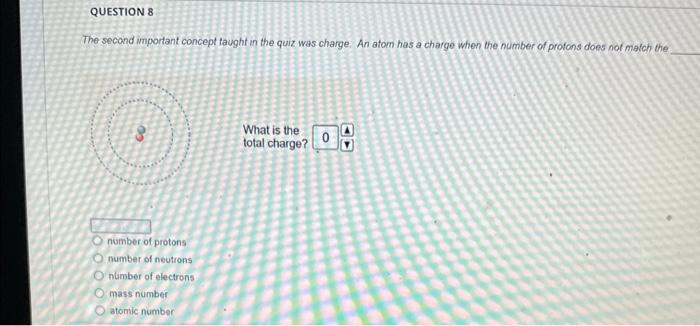 The magnesium isotope represented by the nuclide symbol contains protons, neutrons, and electrons. Protons: Neutrons: Eloctrons: 12, 12,12 12, 12, 13 12. 13.12 13. 12,12 The mass number is What is the mass number? The mass number is Protons: 8 Neutrons: 10 Electrons: 10 The mass number is Protons: 16 Neutrons: 16 Electrons: 16 The number in the top left comer of the nuclide symbol is called the atomic number called the mass number equal to the charge 2 The quiz described a common notation used with atoms. The symbol to define an atom, in the format shown, is called Often, this symbol is written without the charge, such as: zA an orbital diagram an ion symbol a nuclide symbol an electron configuration What is the answer to this question? What is the total charge? The second important concept taught in the quiz was charge. An atom has a charge when the number of protons does not match the What is the total charge? number of protons number of neutrons nimber of electrons mass number atomic number
The magnesium isotope represented by the nuclide symbol contains protons, neutrons, and electrons. Protons: Neutrons: Eloctrons: 12, 12,12 12, 12, 13 12. 13.12 13. 12,12 The mass number is What is the mass number? The mass number is Protons: 8 Neutrons: 10 Electrons: 10 The mass number is Protons: 16 Neutrons: 16 Electrons: 16 The number in the top left comer of the nuclide symbol is called the atomic number called the mass number equal to the charge 2 The quiz described a common notation used with atoms. The symbol to define an atom, in the format shown, is called Often, this symbol is written without the charge, such as: zA an orbital diagram an ion symbol a nuclide symbol an electron configuration What is the answer to this question? What is the total charge? The second important concept taught in the quiz was charge. An atom has a charge when the number of protons does not match the What is the total charge? number of protons number of neutrons nimber of electrons mass number atomic number
please help me out. thank you so much. 








Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


