please help me solve these questions
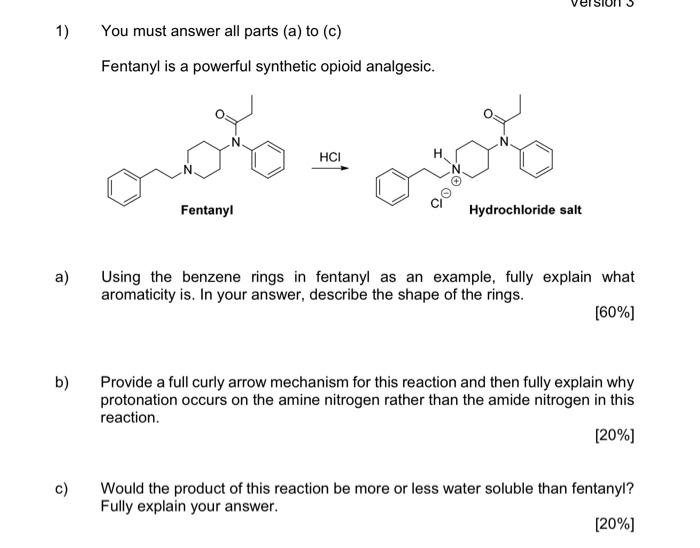
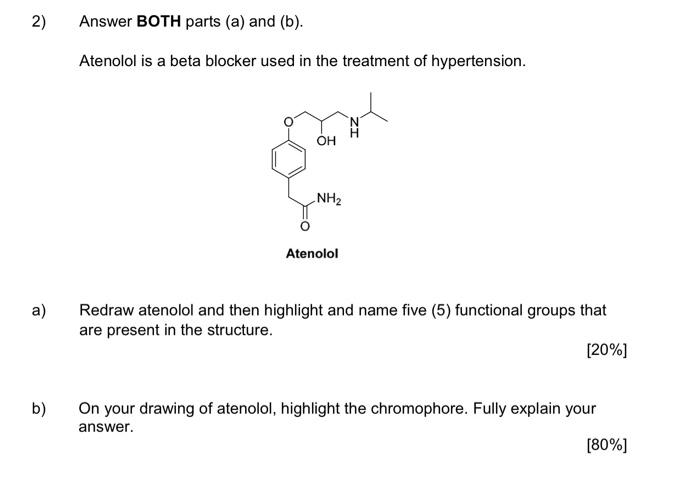
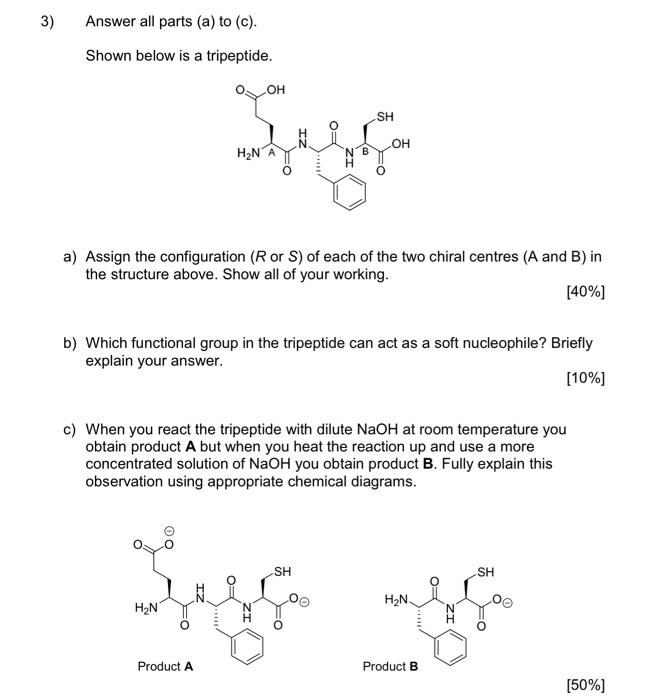
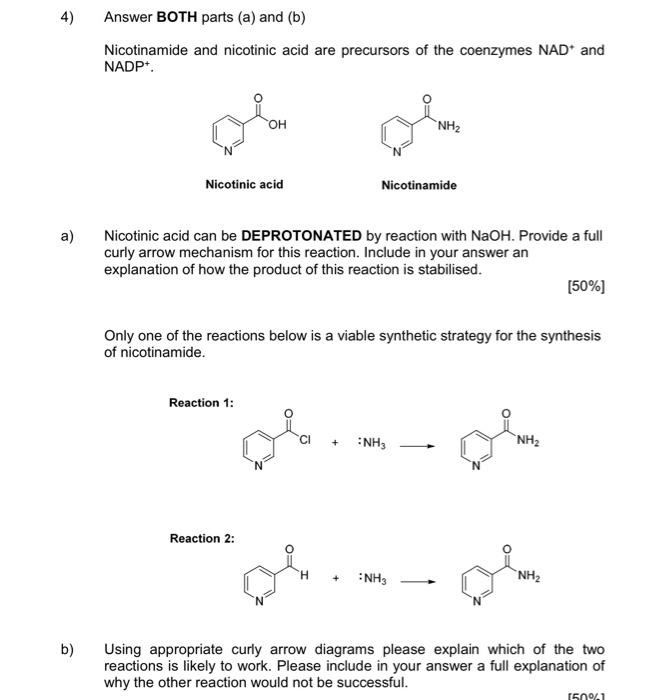
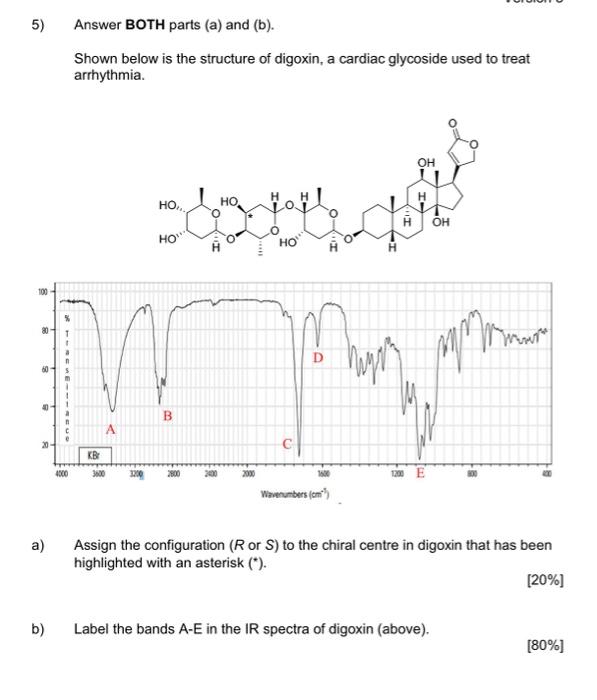
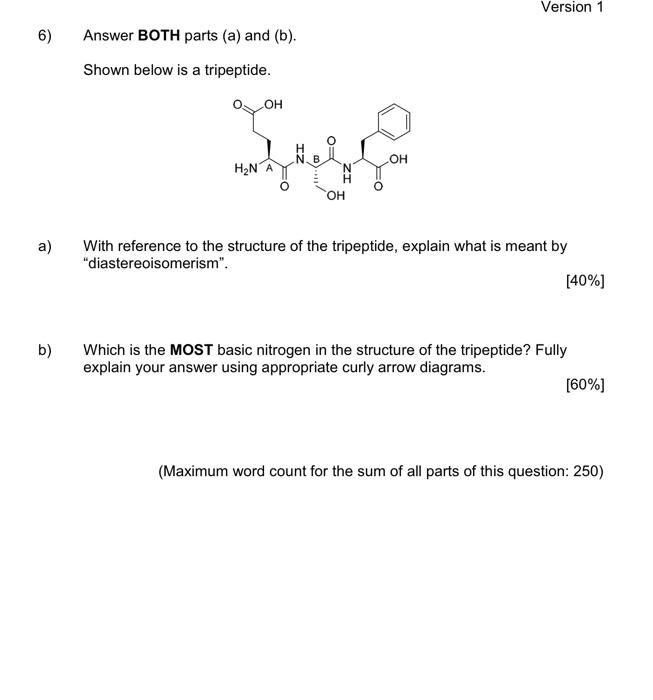
1) You must answer all parts (a) to (c) Fentanyl is a powerful synthetic opioid analgesic. HCI co Fentanyl Hydrochloride salt a) Using the benzene rings in fentanyl as an example, fully explain what aromaticity is. In your answer, describe the shape of the rings. [60%] b) Provide a full curly arrow mechanism for this reaction and then fully explain why protonation occurs on the amine nitrogen rather than the amide nitrogen in this reaction. (20%) c) Would the product of this reaction be more or less water soluble than fentanyl? Fully explain your answer. [20%) 2) Answer BOTH parts (a) and (b). Atenolol is a beta blocker used in the treatment of hypertension. OH NH2 Atenolol a) Redraw atenolol and then highlight and name five (5) functional groups that are present in the structure. [20%] b) On your drawing of atenolol, highlight the chromophore. Fully explain your answer. [80%] 3) Answer all parts (a) to (c). Shown below is a tripeptide. OH SH IZ OH H2N ZI a) Assign the configuration (R or S) of each of the two chiral centres (A and B) in the structure above. Show all of your working. [40%) b) Which functional group in the tripeptide can act as a soft nucleophile? Briefly explain your answer. (10%) c) When you react the tripeptide with dilute NaOH at room temperature you obtain product A but when you heat the reaction up and use a more concentrated solution of NaOH you obtain product B. Fully explain this observation using appropriate chemical diagrams. SH SH H2N H2N Product A Product B (50%) 4) Answer BOTH parts (a) and (b) Nicotinamide and nicotinic acid are precursors of the coenzymes NAD* and NADP+ ooo OH NH2 Nicotinic acid Nicotinamide a) Nicotinic acid can be DEPROTONATED by reaction with NaOH. Provide a full curly arrow mechanism for this reaction. Include in your answer an explanation of how the product of this reaction is stabilised. [50%) Only one of the reactions below is a viable synthetic strategy for the synthesis of nicotinamide. Reaction 1: NH3 NH2 Reaction 2: NH3 NH2 ) b) Using appropriate curly arrow diagrams please explain which of the two reactions is likely to work. Please include in your answer a full explanation of why the other reaction would not be successful. 15041 5) Answer BOTH parts (a) and (b). Shown below is the structure of digoxin, a cardiac glycoside used to treat arrhythmia. OH I HO HO o OH - HO 100 - 80 T D my 20 B A C KB 4000 3600 2200 2800 2000 203 120 E Waverumbers (cm) a) Assign the configuration (R or S) to the chiral centre in digoxin that has been highlighted with an asterisk (*). [20%) b) Label the bands A-E in the IR spectra of digoxin (above). [80%] Version 1 6) Answer BOTH parts (a) and (b). Shown below is a tripeptide. OH See OH H2N OH a) With reference to the structure of the tripeptide, explain what is meant by "diastereoisomerism". [40%) b) Which is the MOST basic nitrogen in the structure of the tripeptide? Fully explain your answer using appropriate curly arrow diagrams. [60%) (Maximum word count for the sum of all parts of this question: 250)