please help me to write 15 reactions.

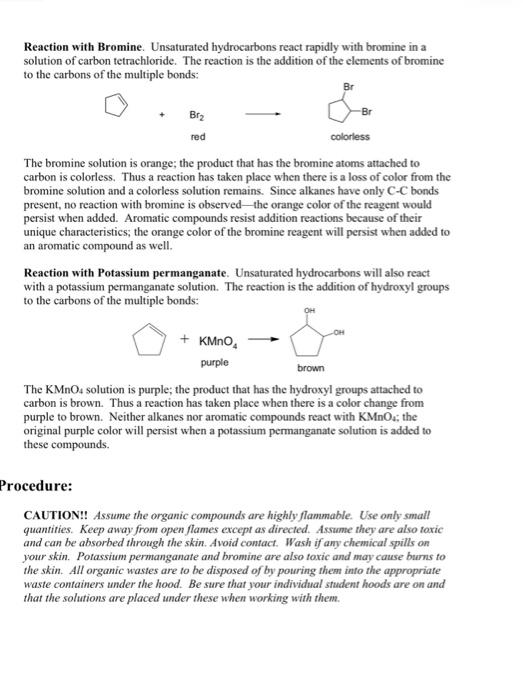
Chemical reactions - write chemical equations for all of the tests performed (combustion, bromine, potassium permanganate), balanced when feasible. If the compound failed to react, indicate this by writing "N.R." for no reaction. Use the structural formula for the known compounds (hexane, cyclohexene, toluene), and just the unknown number with a description of what happened for the unknowns. 3 tests 5 substances =15 equations required. Identification of Hydrocarbons Purpose: To explore simple laboratory tests that can distinguish between the different classes of hydrocarbons. In this experiment the exploration of hydrocarbons is limited to saturated and unsaturated hydrocarbons. Alkanes have the highest ratio of hydrogen to carbon allowed in organic compounds, while unsaturated compounds have a lower ratio of hydrogen to carbon. Formulas for these ratios are found in the lecture textbook. Compounds with a lower ratio of hydrogen to carbon have structural differences - multiple bonds between carbon atoms, or cyclic structures. These structural differences are the keys to differentiating between the different types of hydrocarbons. The simple tests outlined below are designed specifically for the type of unsaturation caused by carbon-carbon multiple bonds. If the test fails, then one can conclude that the hydrocarbon is probably saturated. Background Information: The number of known organic compounds total into the millions. Of these compounds the simplest types are those that contain only hydrogen and carbon atoms. These are known as hydrocarbons. Because of the number and variety of hydrocarbons that exist, some means of classification is necessary. One means of classification depends on the way in which carbon atoms are connected. Aliphatic hydrocarbons are compounds consisting of carbons bonded either in a single chain or in a branched chain. Cyclic hydrocarbons are compounds having the carbon atoms bonded in a closed polygon. For example, n-hexane and 2 -methylpentane are aliphatic molecules, while cyclopentane is a cyclic system: Another means of classification depends on the type of bonding that exists between carbons. Hydrocarbons which contain only carbon-to-carbon single bonds are called alkanes. They are also referred to as saturated molecules. Hydrocarbons containing at least one carbon-to-carbon double bond are called alkenes, and those compounds with at least one carbon-to-carbon triple bond are alkynes. Alkenes and alkynes are also referred to as unsaturated hydrocarbons. Finally a special class of cyclic hydrocarbons, which have a high degree of unsaturation and a unique set of reactions, are called aromatic. The following are examples of each of the above categories: CH3CH2CH2CH3CH3CH2CH2CH3CH21-pentane(alkene)CH3CH2CH2CCH In looking at the types of bonds which each of these compounds contains, one realizes that all of these molecules are nonpolar. Recall that the electronegativity difference between carbon and hydrogen is only 0.4 and that qualifies this bond as a nonpolar bond. In general, hydrocarbons do not mix with polar solvents such as water or ethyl alcohol. On the other hand, hydrocarbons do mix with relatively nonpolar solvents such as ligroin (a mixture of alkanes), carbon tetrachloride or dichloromethane. Since the density of most carbon-hydrogen containing compounds is less than that of water, they will float. Crude oil and crude oil products (home heating, oil and gasoline) are mixtures of hydrocarbons; these substances, when spilled on water, spread quickly along the surface because they do not mix with water and float. The type of bond in the compound determines the chemical reactivity of hydrocarbons. While saturated hydrocarbons, alkanes, will burn (undergo combustion), they are generally unreactive to most reagents. Unsaturated hydrocarbons, alkenes and alkynes, not only burn, but also react by addition of reagents to the double or triple bonds. The addition products become saturated, with fragments of the reagent becoming attached to the carbons of the multiple bonds. Aromatic compounds, with a higher carbon to hydrogen ratio than nonaromatic compounds, burn with a sootier flame as a result of unburned carbon particles being present. Aromatic compounds are resistant to addition reactions. Combustion: The major component in "natural gas" is the hydrocarbon methane. Other hydrocarbons used for heating or cooling purposes are propane and butane. The products from 100% combustion are carbon dioxide and water: 2CH3CH2CH2CH3+13O28CO2+10H2O Reaction with Bromine. Unsaturated hydrocarbons react rapidly with bromine in a solution of carbon tetrachloride. The reaction is the addition of the elements of bromine to the carbons of the multiple bonds: The bromine solution is orange; the product that has the bromine atoms attached to carbon is colorless. Thus a reaction has taken place when there is a loss of color from the bromine solution and a colorless solution remains. Since alkanes have only CC bonds present, no reaction with bromine is observed - the orange color of the reagent would persist when added. Aromatic compounds resist addition reactions because of their unique characteristics; the orange color of the bromine reagent will persist when added to an aromatic compound as well. Reaction with Potassium permanganate. Unsaturated hydrocarbons will also react with a potassium permanganate solution. The reaction is the addition of hydroxyl groups to the carbons of the multiple bonds: The KMnO4 solution is purple; the product that has the hydroxyl groups attached to carbon is brown. Thus a reaction has taken place when there is a color change from purple to brown. Neither alkanes nor aromatic compounds react with KMnO4; the original purple color will persist when a potassium permanganate solution is added to these compounds. rocedure: CAUTION!! Assume the organic compounds are highly flammable. Use only small quantities. Keep away from open flames except as directed. Assume they are also toxic and can be absorbed through the skin. Avoid contact. Wash if any chemical spills on your shin. Potassium permanganate and bromine are also toxic and may cause burns to the skin. All organic wastes are to be disposed of by pouring them into the appropriate waste containers under the hood. Be sure that your individual student hoods are on and that the solutions are placed under these when working with them. Chemicals and Equipment: Most of the chemicals are reagents will be found in dropper bottles in the hood. Bromine will also be found in the hood. Obtain unknowns from the instructor. Each student will have two unknowns that must be classified as alkanes, alkenes or aromatic compounds. The hydrocarbons hexane, cyclohexene and toluene will be available to perform the tests on and record observations from which the chemical classification of the unknowns may be deduced. Physical Properties of Hydrocarbons: Mix approximately 1.0mL (20 drops) of hydrocarbon with 1.0mL of the solvent. Use your small test tubes for these tests. When mixing the compounds, grip the test tube between thumb and forefinger. It should be held firmly enough to keep from slipping but loosely enough so that when the third and fourth fingers tap it, the contents will be agitated enough to mix. Watch for formation of layers, appearance of cloudiness, or oily droplets that do not disappear. All the above indicate that the two substances are not miscible. 1. Water solubility of hydrocarbons. Label your small test tubes with the name of the substance to be tested: Hexane, cyclohexene, toluene, unknown A, and unknown B. Place 20 drops of the appropriate hydrocarbon in each test tube, then add 20 drops of water. Mix the contents as described above and observe the result. Is there separation of layers? Which component is on the bottom and which is on the top? What can you conclude about the solubility of the hydrocarbons and unknowns in water? To further verify the results, record the densities of each of the known hydrocarbons by looking them up in The Merck Index. Record these values, along with the proper reference, in the data section of your lab notebook. 2. Solubility of hydrocarbons in CH2Cl2. Repeat the test as described above, except use 20 drops of dichloromethane as the solvent. Mix the contents and observe the results. Compare these test tubes to those from part 1 and clearly describe your observations. Comment on the solubility of the compounds in this solvent. Is this what you would have expected? Why or why not? Chemical Properties of Hydrocarbons: 1. Combustion. Perform this test in the hood designated for combustion. Do not ignite your samples anywhere else in the lab. Place about ten drops of each compound to be tested on a small watch glass or crucible. Carefully ignite each sample with a match. Observe the flame, color of the smoke, and amount of residue for each of the compounds. Record your observations. Perform this test on hexane, cyclohexene, toluene, unknown A, and unknown B. 2. Reaction with bromine. Label five clean, dry test tubes with the name of the substance to be tested. Place five drops of hexane, cyclohexene, toluene, unknown A, and unknown B in the appropriately labeled test tube. Under the hood carefully add the 1% bromine in CH2Cl2 solution, dropwise with shaking. Keep track in your notebook the number of drops added and any color changes, or other observations; it should not be necessary to add any more than ten drops of the bromine solution. 3.Reaction with potassium permanganate. Label five clean, dry test tubes with the name of the substance to be tested. Place five drops of hexane, cyclohexene, toluene, unknown A, and unknown B in the appropriately labeled test tube. Carefully add the 1% aqueous KMnO4 solution, dropwise with shaking. Keep track in your notebook the number of drops added and any color changes, or other observations; it should not be necessary to add more than ten drops of the KMnO4 solution. Identification of Unknowns: By comparing the observations you made for your unknowns with those of the known hydrocarbons, you can classify unknowns A and B (don't forget to record the number on the side of the vials somewhere) as alkanes, alkenes or aromatic compounds. Record their classifications and offer a brief explanation for your choice