Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me When 280mg of a certain molecular compound X are dissolved in 45.5 of dibenzyl ether (CH, CH), ), the freezing point of
please help me 
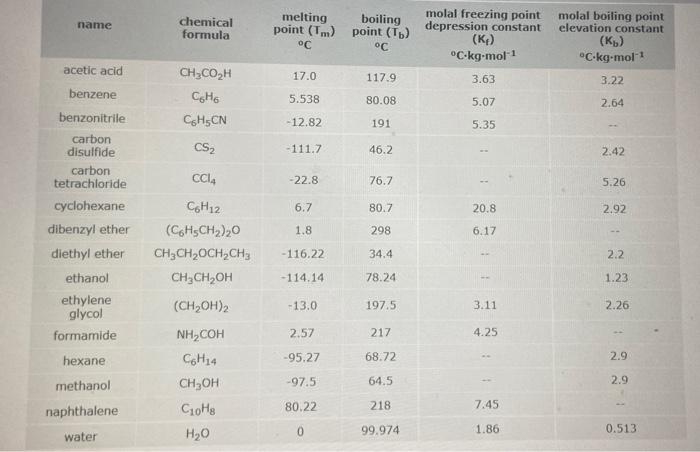
When 280mg of a certain molecular compound X are dissolved in 45.5 of dibenzyl ether (CH, CH), ), the freezing point of the solution is measured to be 1.2 Calolate the molar mass of X If you need any additional information on dibercryl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and founded to significant digit 0 B OP X 5 2 2 name chemical formula melting boiling point (Tm) point (T) C C molal freezing point depression constant (Kp) C-kg-mol-1 molal boiling point elevation constant () C-kg-mol-1 3.22 acetic acid 17.0 117.9 3.63 CH3COOH GHO CHECN 5.538 80.08 5.07 2.64 - 12.82 191 5.35 CS2 -111.7 46.2 2.42 CCIA -22.8 76.7 5.26 6.7 80.7 20.8 2.92 benzene benzonitrile carbon disulfide carbon tetrachloride cyclohexane dibenzyl ether diethyl ether ethanol ethylene glycol formamide 1.8 298 6.17 -116.22 34.4 2.2 -114.14 78.24 1 1.23 -13.0 197.5 3.11 2.26 CH12 (C6H3CH2), CH3CH2OCH,CHE CH3CH OH (CH2OH)2 NHCOH Colia , Coto H2O 2.57 217 4.25 hexane -95.27 68.72 2.9 -97.5 64.5 2.9 methanol naphthalene 80.22 218 7.45 0 99.974 1.86 water 0.513 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


