Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help me with these questions, thank you very much! What of the following options describes the term polarizability? a. Capability of a mixture to
Please help me with these questions, thank you very much! 






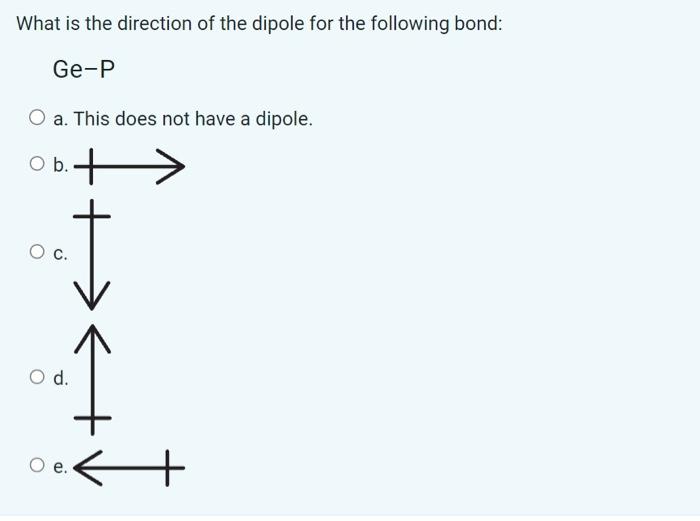


What of the following options describes the term "polarizability"? a. Capability of a mixture to form a single phase. b. The ease of distortion of the electron cloud of a molecular entity by an electric field. c. A stronger form of dipole-dipole interaction between an electronegative atom and a hydrogen atom attached to a second, relatively electronegative atom. Both electronegative atoms are usually N,O or F. d. The attractive or repulsive forces between molecular entities (or between groups within the same molecular entity) including dipole-dipole and dispersion forces. e. The attractive interaction between the negatively-charged end of a polar molecule (or group) with the positively-charged end of a neighbouring polar molecule (or group). Consider a pure sample of chlorine trifluoride which has the formula ClF3. Of the options listed below, what is the strongest attractive force in this sample? a. Dipole-dipole forces b. Ionic bonding c. Ion-dipole forces d. Hydrogen bonding e. Dispersion forces Consider the following 2 molecules: selenium tetrafluoride which has the molecular formula SeF4, and ethane, an alkane which has the molecular formula C2H6. Which of these molecules is polar? a. Neither are polar. b. Selenium tetrafluoride is polar but ethane is not. c. Ethane is polar but selenium tetrafluoride is not. d. Both are polar. Consider the three compounds: methanol (CH3OH), acetic acid (CH3COOH), and pentane (C5H12). Which of the options below describes their miscibility? a. Acetic acid and methanol are miscible with each other, but neither are miscible with pentane. b. All three compounds are miscible. c. None of the compounds are miscible with any other compounds given. d. Acetic acid and pentane are miscible with each other, but neither are miscible with methanol. e. Pentane and methanol are miscible with each other, but neither are miscible with acetic acid. The partial pressure of O2 in the atmosphere at sea level is 21.3kPa. What is the partial pressure of O2 on the top of a mountain, where the atmospheric pressure is 62.7kPa ? [Atmospheric pressure at sea level is 101.3kPa.] a. 34.4kPa b. 13.2kPa c. 62.7kPa d. 21,3kPa e. 1.8kPa A sample of gas at 972K with a volume of 14.8L undergoes a temperature change which changes to volume to 33.8L. What is the new temperature of the gas after this change? a. 426K b. 0.515K c. 4.86105K d. 2220K e.1.94 K What intermolecular forces exist between molecules in a pure sample of sulfur hexachloride which has the molecular formula SCl6 ? a. Dispersion forces, dipole-dipole forces and hydrogen bonding. b. Dispersion forces only c. Hydrogen bonding only. d. Dipole-Dipole forces only. e. Dispersion forces and dipole-dipole forces only What is the direction of the dipole for the following bond: Ge-P a. This does not have a dipole. Which of the following lists correctly ranks the species in order of increasing strength of intermolecular forces in a pure sample? a. Cl2









Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


