please help me witj those question
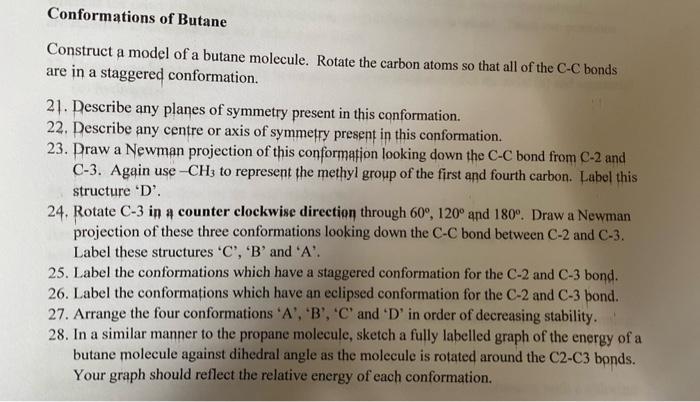
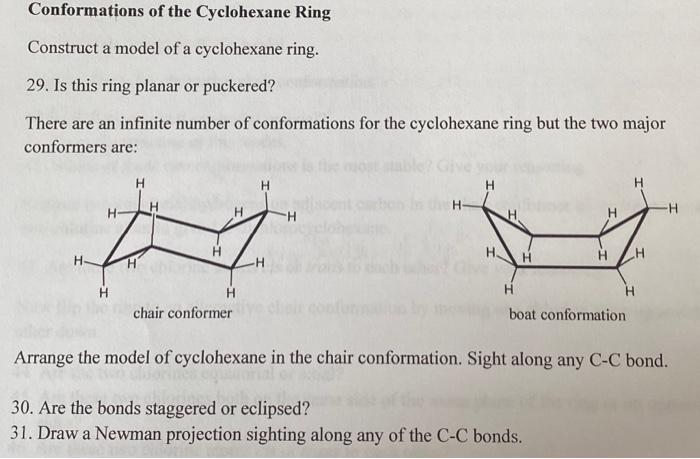
Conformations of Butane Construct a model of a butane molecule. Rotate the carbon atoms so that all of the CC bonds are in a staggered conformation. 21. Describe any planes of symmetry present in this conformation. 22. Describe any centre or axis of symmetry present in this conformation. 23. Praw a Newman projection of this conformation looking down the CC bond from C2 and C-3. Again use CH3 to represent the methyl group of the first and fourth carbon. Label this structure ' D '. 24. Rotate C3 in a counter clockwise direction through 60,120 and 180. Draw a Newman projection of these three conformations looking down the CC bond between C2 and C3. Label these structures ' C ', ' B ' and ' A '. 25. Label the conformations which have a staggered conformation for the C2 and C3 bond. 26. Label the conformations which have an eclipsed conformation for the C2 and C3 bond. 27. Arrange the four conformations ' A ', ' B ', ' C ' and ' D ' in order of decreasing stability. 28. In a similar manner to the propane molecule, sketch a fully labelled graph of the energy of a butane molecule against dihedral angle as the molecule is rotated around the C2C3 bonds. Your graph should reflect the relative energy of each conformation. Conformations of the Cyclohexane Ring Construct a model of a cyclohexane ring. 29. Is this ring planar or puckered? There are an infinite number of conformations for the cyclohexane ring but the two major conformers are: Arrange the model of cyclohexane in the chair conformation. Sight along any C-C bond. 30. Are the bonds staggered or eclipsed? 31. Draw a Newman projection sighting along any of the C-C bonds. At each carbon atom one CH bond lies approximately in the mean plane of the molecule, whereas the other CH bond is approximately perpendicular to the plane. These two positions are referred to as equatorial and axial respectively. 32. Draw a cyclohexane molecule in the chair conformation and label the axial (a) and equatorial (e) hydrogens 33. How many equatorial hydrogens are present? 34. How many axial hydrogens are a. Above the mean plane of the ring? b. Below the mean plane of the ring? 35. What pattern is thee for the axial hydrogens? Arrange the model of cyclohexane in the boat conformation. Sight along the two C-C bonds which make up the 'sides' of the boat. 36. Are these bonds staggered or eclipsed? 37. Draw a Newman projection for the C-C bond in one side of the boat. 38. Which of these two conformations of cyclohexane is the most stable? Give your reasoning. Conformations of Butane Construct a model of a butane molecule. Rotate the carbon atoms so that all of the CC bonds are in a staggered conformation. 21. Describe any planes of symmetry present in this conformation. 22. Describe any centre or axis of symmetry present in this conformation. 23. Praw a Newman projection of this conformation looking down the CC bond from C2 and C-3. Again use CH3 to represent the methyl group of the first and fourth carbon. Label this structure ' D '. 24. Rotate C3 in a counter clockwise direction through 60,120 and 180. Draw a Newman projection of these three conformations looking down the CC bond between C2 and C3. Label these structures ' C ', ' B ' and ' A '. 25. Label the conformations which have a staggered conformation for the C2 and C3 bond. 26. Label the conformations which have an eclipsed conformation for the C2 and C3 bond. 27. Arrange the four conformations ' A ', ' B ', ' C ' and ' D ' in order of decreasing stability. 28. In a similar manner to the propane molecule, sketch a fully labelled graph of the energy of a butane molecule against dihedral angle as the molecule is rotated around the C2C3 bonds. Your graph should reflect the relative energy of each conformation. Conformations of the Cyclohexane Ring Construct a model of a cyclohexane ring. 29. Is this ring planar or puckered? There are an infinite number of conformations for the cyclohexane ring but the two major conformers are: Arrange the model of cyclohexane in the chair conformation. Sight along any C-C bond. 30. Are the bonds staggered or eclipsed? 31. Draw a Newman projection sighting along any of the C-C bonds. At each carbon atom one CH bond lies approximately in the mean plane of the molecule, whereas the other CH bond is approximately perpendicular to the plane. These two positions are referred to as equatorial and axial respectively. 32. Draw a cyclohexane molecule in the chair conformation and label the axial (a) and equatorial (e) hydrogens 33. How many equatorial hydrogens are present? 34. How many axial hydrogens are a. Above the mean plane of the ring? b. Below the mean plane of the ring? 35. What pattern is thee for the axial hydrogens? Arrange the model of cyclohexane in the boat conformation. Sight along the two C-C bonds which make up the 'sides' of the boat. 36. Are these bonds staggered or eclipsed? 37. Draw a Newman projection for the C-C bond in one side of the boat. 38. Which of these two conformations of cyclohexane is the most stable? Give your reasoning