Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help :) Monoprotic acids Acetic acid (K-174 x 10-5 CHACO CH, CH 0 OH K-476 Ammonium ion 06-562 X 100) NH K-925 Diprotic acids
please help :) 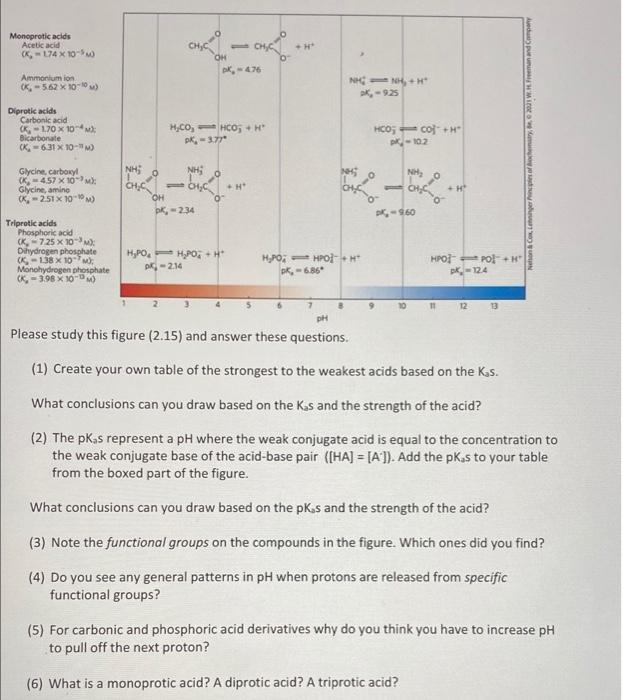 Monoprotic acids Acetic acid (K-174 x 10-5 CHACO CH, CH 0 OH K-476 Ammonium ion 06-562 X 100) NH K-925 Diprotic acids Carbonic acid - 170 X 10- Bicarbonate (K. - 631 10- Hico, - HCO; +H PK, 3.77 HCO3 =CO+H K-102 Norge, W. Freeman and Comment , NH NH Glycine, carboxy! K457 x 10 Glycine, amine - 2.51 X 100 long H' loth + 0 OH DK, -234 O - 960 Tripratic acids Phosphoric acid K-725 x 10- Dihydrogen phosphate - 138 x 10 Monohydrogen phosphate K-398 X 100 H,POH POH - 2.14 H,POHOH K.-6.86 HPOT Pol+ pk-124 7 13 ph Please study this figure (2.15) and answer these questions. (1) Create your own table of the strongest to the weakest acids based on the Ks. What conclusions can you draw based on the Ks and the strength of the acid? (2) The pK, s represent a pH where the weak conjugate acid is equal to the concentration to the weak conjugate base of the acid-base pair ([HA] = [A]). Add the pKas to your table from the boxed part of the figure. What conclusions can you draw based on the pks and the strength of the acid? (3) Note the functional groups on the compounds in the figure. Which ones did you find? (4) Do you see any general patterns in pH when protons are released from specific functional groups? (5) For carbonic and phosphoric acid derivatives why do you think you have to increase pH to pull off the next proton? (6) What is a monoprotic acid? A diprotic acid? A triprotic acid
Monoprotic acids Acetic acid (K-174 x 10-5 CHACO CH, CH 0 OH K-476 Ammonium ion 06-562 X 100) NH K-925 Diprotic acids Carbonic acid - 170 X 10- Bicarbonate (K. - 631 10- Hico, - HCO; +H PK, 3.77 HCO3 =CO+H K-102 Norge, W. Freeman and Comment , NH NH Glycine, carboxy! K457 x 10 Glycine, amine - 2.51 X 100 long H' loth + 0 OH DK, -234 O - 960 Tripratic acids Phosphoric acid K-725 x 10- Dihydrogen phosphate - 138 x 10 Monohydrogen phosphate K-398 X 100 H,POH POH - 2.14 H,POHOH K.-6.86 HPOT Pol+ pk-124 7 13 ph Please study this figure (2.15) and answer these questions. (1) Create your own table of the strongest to the weakest acids based on the Ks. What conclusions can you draw based on the Ks and the strength of the acid? (2) The pK, s represent a pH where the weak conjugate acid is equal to the concentration to the weak conjugate base of the acid-base pair ([HA] = [A]). Add the pKas to your table from the boxed part of the figure. What conclusions can you draw based on the pks and the strength of the acid? (3) Note the functional groups on the compounds in the figure. Which ones did you find? (4) Do you see any general patterns in pH when protons are released from specific functional groups? (5) For carbonic and phosphoric acid derivatives why do you think you have to increase pH to pull off the next proton? (6) What is a monoprotic acid? A diprotic acid? A triprotic acid

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


