Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help Part A Write condensed electron configurations and orbital diagrams for each element: Write condensed electron configuration for S. Express your answer in condensed
please help 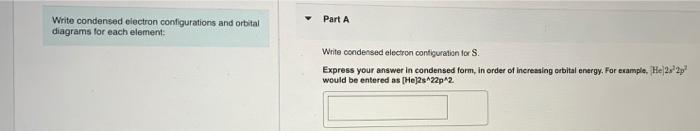
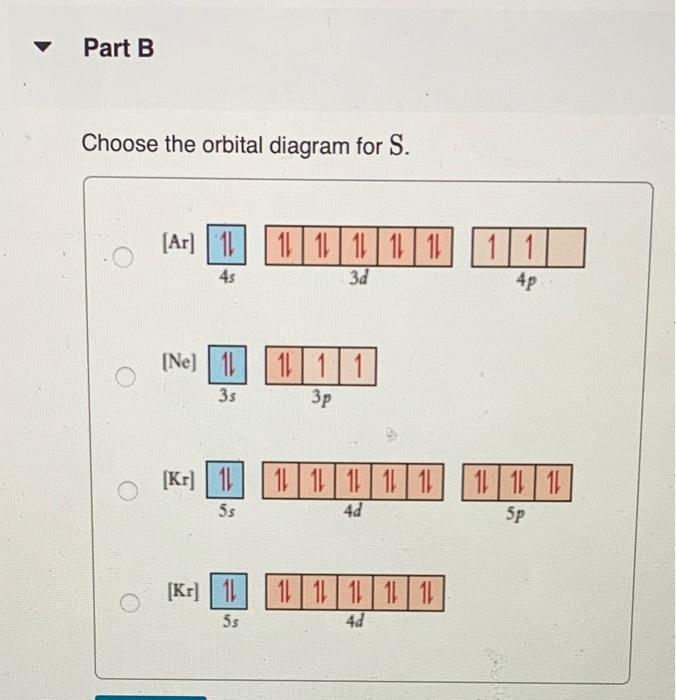
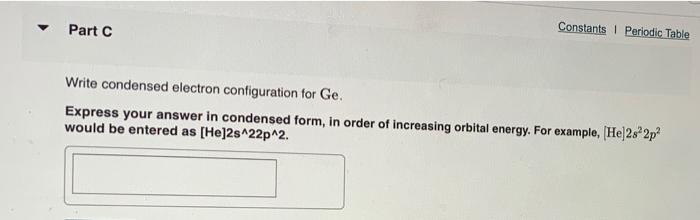
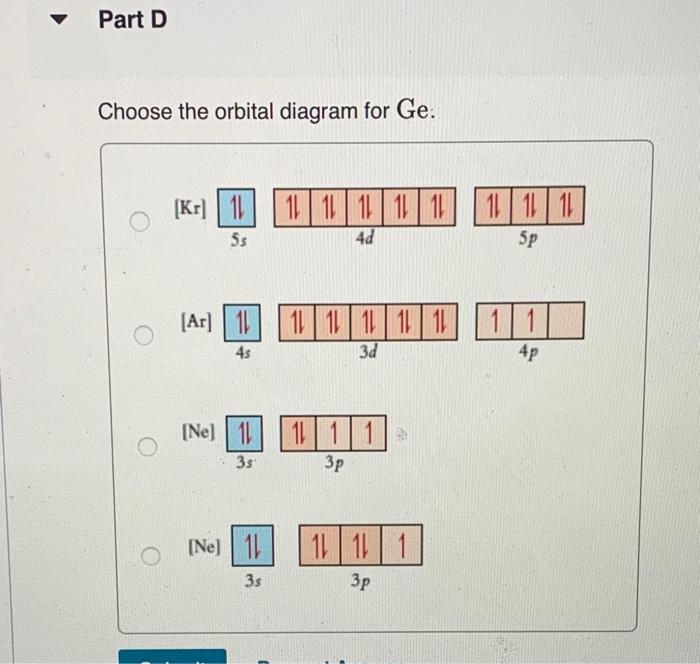
Part A Write condensed electron configurations and orbital diagrams for each element: Write condensed electron configuration for S. Express your answer in condensed form, in order of increasing orbital energy. For example. He|222 would be entered as [Hej2s22p2 Part B. Choose the orbital diagram for S. [| [Ar] 111111111111 4p 45 3d [Ne) 11 35 1111 3p [Kr] 111111111111 ) 1111111 5s 4d 5p [Kr] 111111111111 5$ 4d Part C Constants | Periodic Table Write condensed electron configuration for Ge. Express your answer in condensed form, in order of increasing orbital energy. For example, (He]2s2p? would be entered as [He]2s^22p^2. Part D D Choose the orbital diagram for Ge. [Kr) 1 5s 1111111111111111 4d 5p [Ar] 111111111 45 3d 4p 11 [ [Ne) 10 11 3s 3p [Ne) 11 11 11 3s 3p 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


