Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please help Problem 2: The common amino acids are polyprotic acids. All amino acids contain an amino group and a carboxyl group. Some amino acids
Please help 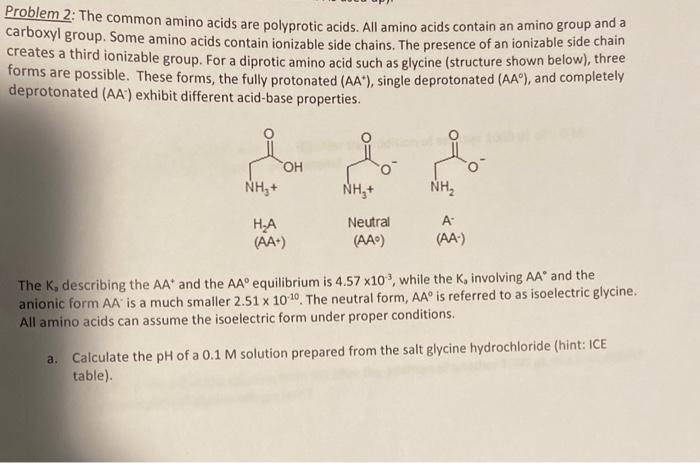
Problem 2: The common amino acids are polyprotic acids. All amino acids contain an amino group and a carboxyl group. Some amino acids contain ionizable side chains. The presence of an ionizable side chain creates a third ionizable group. For a diprotic amino acid such as glycine (structure shown below), three forms are possible. These forms, the fully protonated (AA"), single deprotonated (AA), and completely deprotonated (AA) exhibit different acid-base properties. NH,+ NH+ NH (AA) Neutral (AA) A (AA) The K, describing the AA and the AA equilibrium is 4.57x10, while the K, involving AA and the anionic form AA is a much smaller 2.51 x 10-10. The neutral form, AA is referred to as isoelectric glycine. All amino acids can assume the isoelectric form under proper conditions. a. Calculate the pH of a 0.1 M solution prepared from the salt glycine hydrochloride (hint: ICE table) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


