Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help Problem 5: In Alberta, ethylene is transported by pipeline at about 10 deg C, 10 MPag. a. Look up the critical properties and


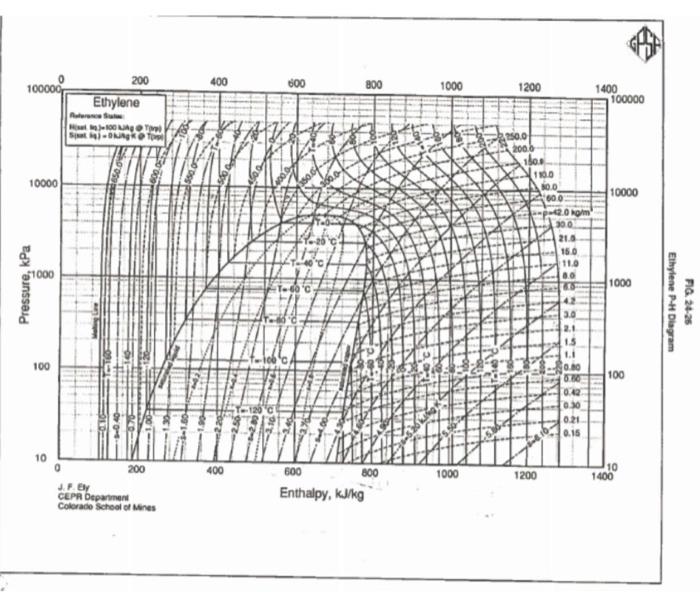
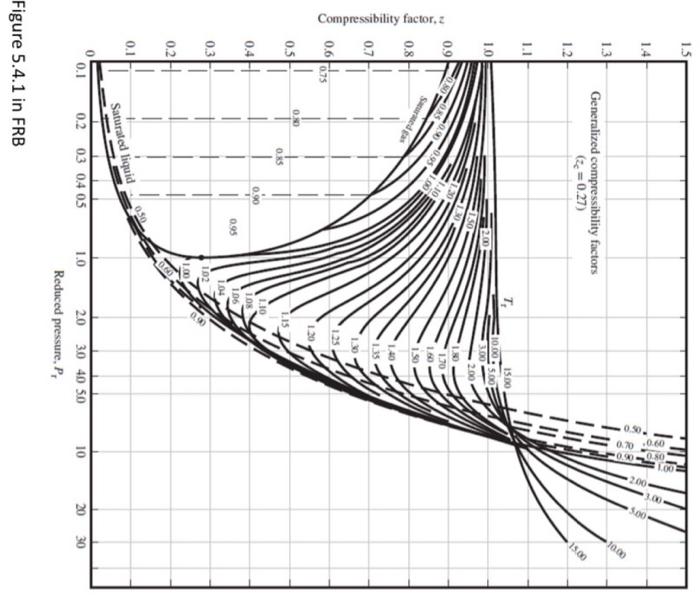
please help
Problem 5: In Alberta, ethylene is transported by pipeline at about 10 deg C, 10 MPag. a. Look up the critical properties and boiling point of ethylene in FRB (or another credible, documented source). Sketch the Liquid, vapour, Supercritical fluid part of the 1 component phase diagram for ethylene (log P vs T) showing this operating point, the normal boiling point, and the critical point. What phase is the operating point ? Explain how you know. In order to design the pipeline system, we need to know the density of the fluid. Parts b through e will look at different methods of determining the density. Tabulate your results for these parts. Title your table, "Density of ethylene at 10degC,10MPag, by different methods". Include 3 Columns in your table: Method, [kg/m3], and Comments. In the "Comment" column, discuss accuracy, precision and ease of use of each method. b. The method of corresponding states ( Tr,Pr and the Compressibility charts in FRB (Figures 5.4). Comment on whether the ideal gas law would be an appropriate tool to determine the density of this fluid. Document which of the 4 charts you used, and explain why. c. Use the attached P-H diagram to determine the density at these conditions. d. Use the NIST Webbook to determine the density of ethylene at these conditions. e. From your work above, which is the 'best' estimate of density? Why? f. Use the Peng-Robinson equation of state (PR EOS) to estimate the pressure corresponding to this temperature and density. (use a==0.085 ). Use the XL spreadsheet "EOS" from the lecture notes as a model. Use what you consider to be the 'best' value of density to estimate the value of V^, m3/mol. Solve for specific volume. Comment on whether the PR EOS is consistent your other methods. P=VbRTV2+2bV2a Where V is the specific volume, [m3/gmol], T,[K] and P[Pa] (both are absolute units), R=8.314Pam3/mol.K abTr=pc0.45724R2Tc2=pc0.07780RTc=(1+(1Tr0.5))2=0.37464+1.542260.269922=TcT Figure 5.4 .1 in FRB Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


