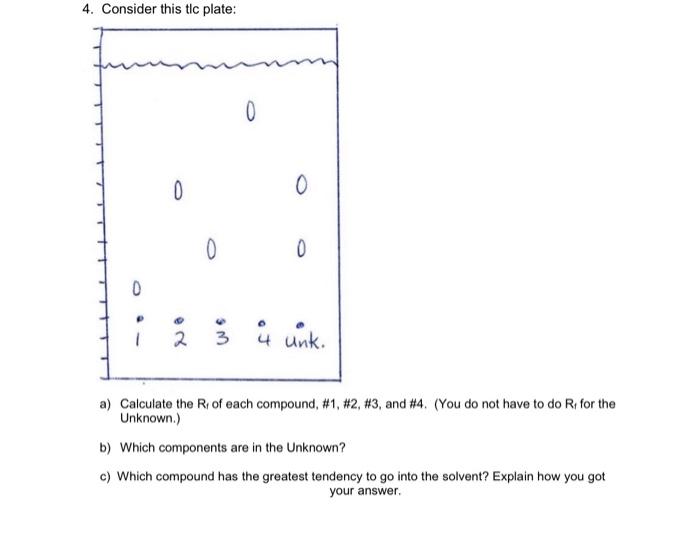
Procedure: 1. Obtain a TLC strip from the supplies counter, handing the strip by the edges being careful not to bend the strip. Using a lead pencil (not a pen) lightly draw a line across the plase about 1.5cm from the botfom (across the short edge). Using a centimeter fuler, lightyy mark ofl six equaly spaced intervals along your line. These are the points at which the samples will be spotted. You will spot one unknown analgesic samples and each of the reference compound solutions containing: acetaminophen, aspirin, catleine, and buproten. Be sure to keep them in alphabetical order. 2. Obtain half a tablet of each of the two unknown anaigesics to be analyzed. Crush the tablets by using a mortar and pestle. Transfer each crushed half-tablet to a simalt, labeled test tube. 3. Add to each test tube about 5mL of a 1:f solution of methylene chloridelethyl alcohol to each of the test tubes and then heat each of them gently for a few minutes in a water bath. The tablets will not completely dissolve due to the insoluble binder, or bullered coaling. 4. After heating the samples, allow them to sette and then spot. the clear liquid extracts on the TLC plate. To spot the plate, dip a micro capillary tube into the extract. Lighty touch the capillary tube to the plase being careful not to dislodge the silica gel. Allow just a small spot of liquid onto the plate. Once the initial spot is dry. repeat the spoling procedure. 5. Nexd spot the four reference solutions, acetaminophen, aspirin, calleine, and ibuprolen, onto the plate in the same manner, using a fresh micro capillary tube for each spot. Acetominophen and ibuprofen will likely be fine with a single spottiny, the aspirin solution should be spothed twice, and the caffeine will require three applications in all. If you are uncertain as to whether yourve gotten enough materiat onto the TLC plate, or if your spots are small enough, preview the undeveloped plate under the UV lamp. 6. Once the plate has been spotted, it is ready to be placed in a 400mL beaker containing about 1cm of the developing solution (ethyl acetale). This beaker should be equipped with a fiter paper liner to help insure an enverment saturated with solvent vapor inside the beakec. Be sure that the developing solution is below the level of your spots and that the TLC plate does not come into direct contact with the fitier paper liner. Afer placing your TLC strip into the beaker, cover it with a watchglass or aluminum foll. 7. When the solvent has risen to a level of about 1cm from the sop of the TLC plate, remove the plate from the chamber and immediately mark the position of the solvent front using a lead pencil. Let the plate dry. When the plate is dry, observe it under a short-wavelength UV lamp. Lighdy outline all the observed spots with a pencil. Put the TLC in a bagsie and attach it to your lab report. 8. Calculate the R, values of each spot using this equation: center of spot R4= line distance from starting line to finish 4. Consider this tlc plate: a) Calculate the Rf of each compound, \#1, \#2, \#3, and \#4. (You do not have to do Rf for the Unknown.) b) Which components are in the Unknown? c) Which compound has the greatest tendency to go into the solvent? Explain how you got your